Today's Human Body Molecule #8: Bicarbonate
Molecular weight: 61
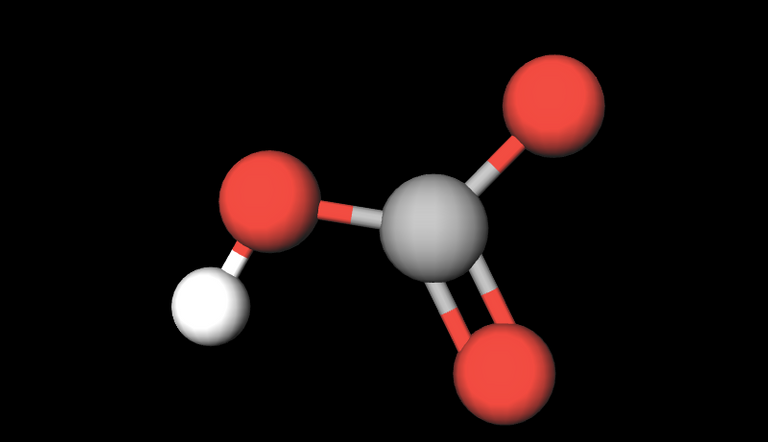
Formula: HCO3-. Conjugate acid: H2CO3
Bicarbonate is one of the most important components of the pH buffering system of the plasma. The pH has to be kept within a very tight range (7.35-7.45) for the proper biochemical functioning of all substances in a living body. Bicarbonate exists in equilibrium with carbonic acid, its conjugate acid (H2CO3) and the relationship between both of them determines the pH of the plasma (or of any liquid controlled by this pH buffering system).
Bicarbonate is generated when carbon dioxide and water react. This reaction is greatly accelerated by an enzyme called carbonic anhydrase.
A reduction in the bicarbonate concentration is the result of a process called metabolic acidosis and an increase in is concentration is the product of metabolic alkalosis.
Bicarbonate, give intravenously or orally can help treat certain conditions related to metabolic acidosis.