Today's Human Body Molecule #3: Carbon Dioxide
Molecular weight: 44
Formula: CO2
Carbon dioxide is a molecule which is known because it is the product of the oxidation of carbohydrates and lipids through respiration to generate energy equivalents. When it dissolves in plasma it forms carbonic acid the relationship between carbonic acid and bicarbonate determine the pH of the plasma, which needs to be kept within a very tight range. Ventilation is the method by which carbon dioxide leaves the body and its accumulation in respiratory illnesses which affect ventilation (COPD, sleep apnea, respiratory depression from narcotics) can cause toxicity which may manifest as encephalopathy.
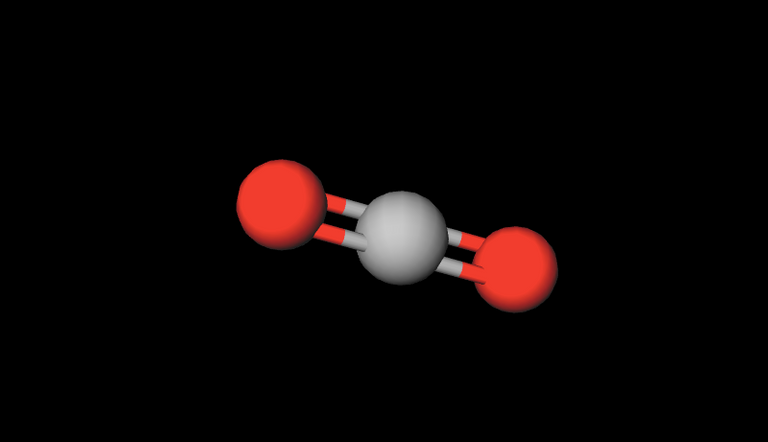
Picture created with molview.org
Gray=Carbon, Red=Oxygen
References
“Carbon dioxide.” Wikipedia The Free Encyclopedia. Wikipedia, December 17, 2019. https://en.wikipedia.org/wiki/Creatinine
Previous Posts in this Series
Today's Human Body Molecule #1: Urea
Today's Human Body Molecule #2: Creatinine
0
0
0.000
The concentration of carbon dioxide in inhaled air is about 0.04% and the concentration of carbon dioxide in exhaled air is about 5%
Yes, for calculation purposes the inhaled carbon dioxide is negligible