CHEMISTRY IN CONTEXT// Toxicity and risk of chemical products

Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of the #hive community who are attracted to information associated with the area of science. This issue serves as the opening of a series of publications that emphasize the usefulness of chemistry as an applied science, where we will describe the scope of this science in our society.
As it has been constant, we will share this publication through the @stemsocial community, who are the best disseminators of contents associated with chemistry, mathematics, engineering, among others. In this opportunity, we will focus on the evaluation of chemical products through the levels of toxicity and risks for the human being.
INTRODUCTION
The life expectancy of our society has increased fivefold since 1800, thanks to the advances made by the ive and the emergence of different substances (medicines, cleaning products and personal hygiene, among others) that have improved the quality of life of society, in addition, the evolutionary process has been such that today we can synthesize substance for crop yields and disease control.
In the same way, we have advanced in the means of transport, we no longer use carriages or horses to move around, thanks to the technological advances, today we have cars and airplanes with a high level of sophistication, where some of the materials used, we obtain them by means of experimental work (for example polymers), whose properties are different from those found in nature and those of the wood and metals we obtain from it.
All the advances mentioned above have, in one way or another, a direct relationship with the emergence of "Chemistry as a science", which we can define as that which is in charge of studying the transformations of matter, understood as everything that surrounds us. Therefore, "Chemistry is in everything.
In this sense, through the following publication we will focus on the study of the levels that are considered as toxic or risky for humanity, starting from the quantitative analysis of the substances.
Fig. 2. Representation of the scopes of chemistry. Author: @madridbg, adapted from. Tomislav Jakupec
CHEMISTRY AS A SCIENCE: FROM QUESTIONING TO EXPERIMENTATION
The most appropriate way to understand the functioning of chemistry as a science is to take a look around us and question the why of the phenomena we observe.
In particular, I am looking at an old tool with a brown coating that many of us call rust, now if we question something so simple we should ask ourselves the following:
1. ¿What is rust?, ¿how is it produced?, ¿what does it have to do with the previous approaches?, ¿where is chemistry present in this phenomenon?
Being objective, these are very simple questions behind a complex phenomenon, but such questions lead us to experimentation, which will allow us to make an interpretation and create a hypothesis that can explain the phenomenon and thus build a theory or a consistent explanation for the moment, which may be subject to change if new aspects arise that the theory cannot justify.

Fig. 3. Representation of the constant oxidation exhibited by iron materials. Author: @madridbg, through Power Point 2010, using public domain images.
Once the questioning is present, we can only demonstrate what is thought with fact, and it is there where experimentation plays a crucial role, where the analysis, processing and control of variables can give an answer to the phenomenon being studied.
CHEMISTRY AS A SCIENCE: EXPERIMENTATION AND MEASUREMENT
In order to understand the toxicity and risks of chemicals, we must first be clear about the units of measurement used in the manufacture of these products. Therefore, we will make a basic overview of these units, which are the starting point in an experimental process.
According to Mcmurry and Fay (2008), the metric system is used in all industrialized countries except the United States and consists of seven fundamental units as we can see in table 1.
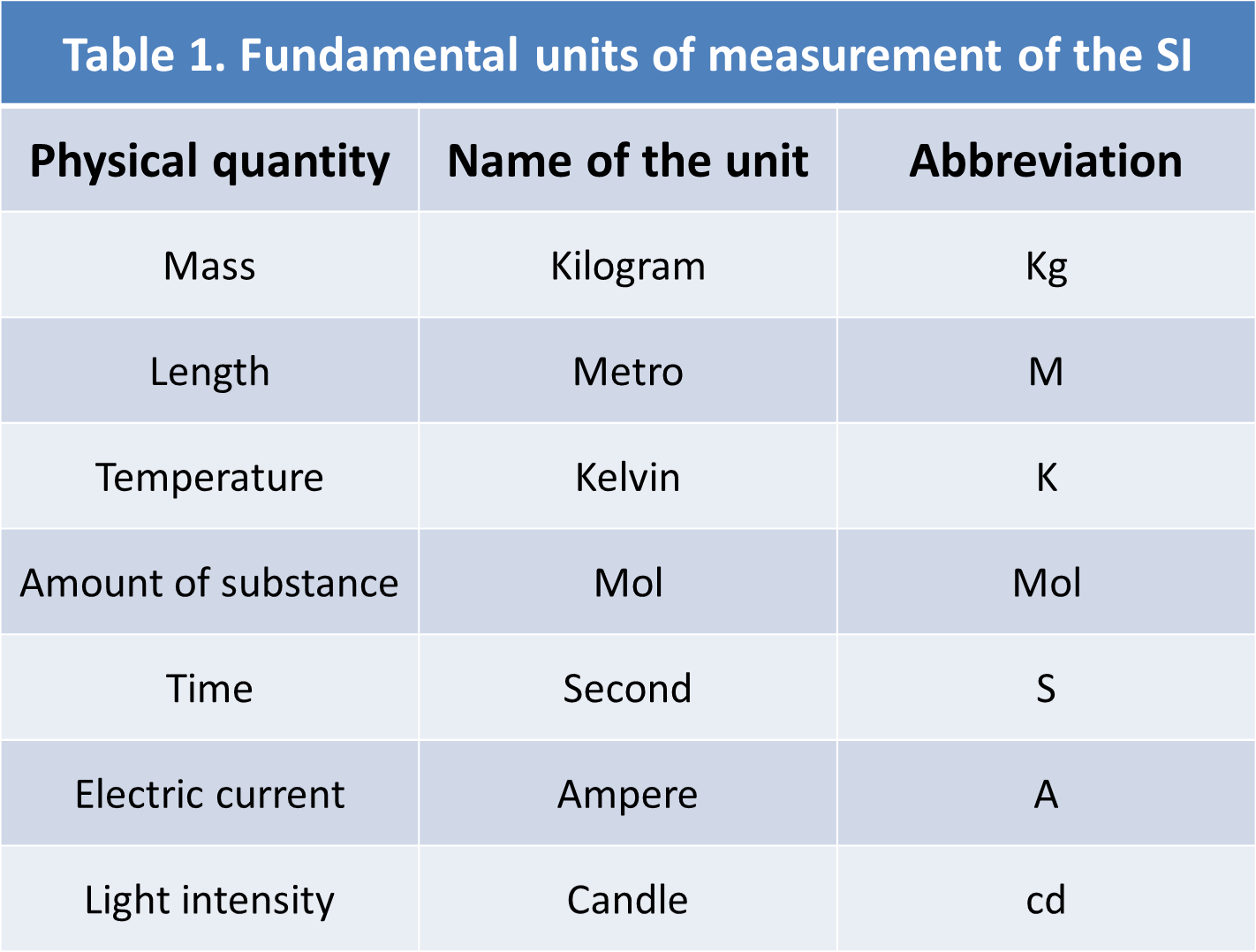
Author: @madridbg, through Power Point 2010. Adapted from: Mcmurry and Fay (2008).
In this regard, we will focus on only one of the seven units shown above, as it is associated with the toxicity of chemicals. Therefore, we will focus our attention on the units of mass and the transformation or variant that these may present.
In relation to the mass, we can define it as the amount of matter that a body possesses, it uses as unit of measurement the kilogram (Kg) and it can be measured with an instrument called Scale.
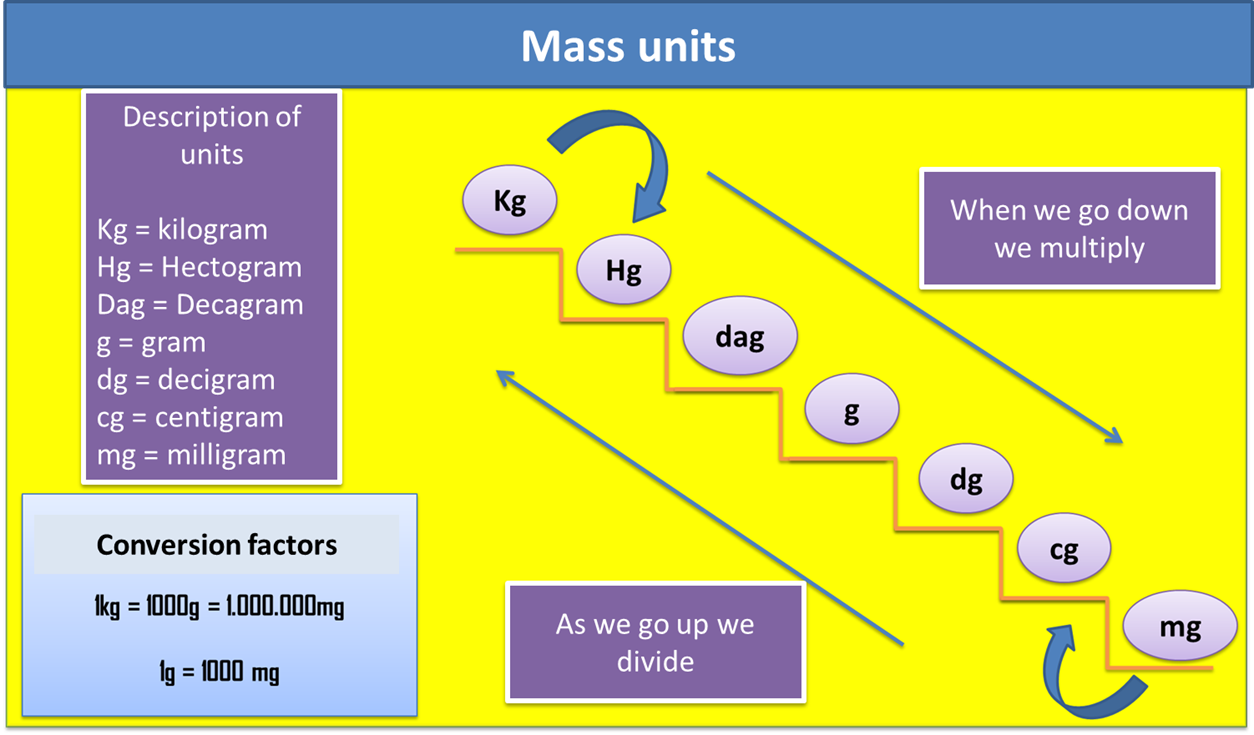
Fig. 4. Representation of mass units. Author: @madridbg, through Power Point 2010.
Therefore, in order to instruct the reader in the use of the mass unit transformations, the following practical approach is proposed:
To respond to this problem we will follow a didactic sequence, which will allow us to instruct the reader in the approach to the problem posed:
1. Locate the units of mass. (see figure 4)
2. Locate the input unit and the output unit proposed by the exercise.
Input unit: is the one that is accompanied by a number, in the proposed exercise it is represented by the 2.5 kg.
Output unit: is the one that we obtain once the input unit has been transformed, in this case it is represented by the mg.
3. We verify in the mass scale, if we go up or down the ladder to know if I should multiply or divide. In this particular case it is multiplied.
4. Verify how many steps I go up or how many steps I go down in the ladder, remember that each step is equivalent to a zero that you must place in the mathematical operation.
Results:
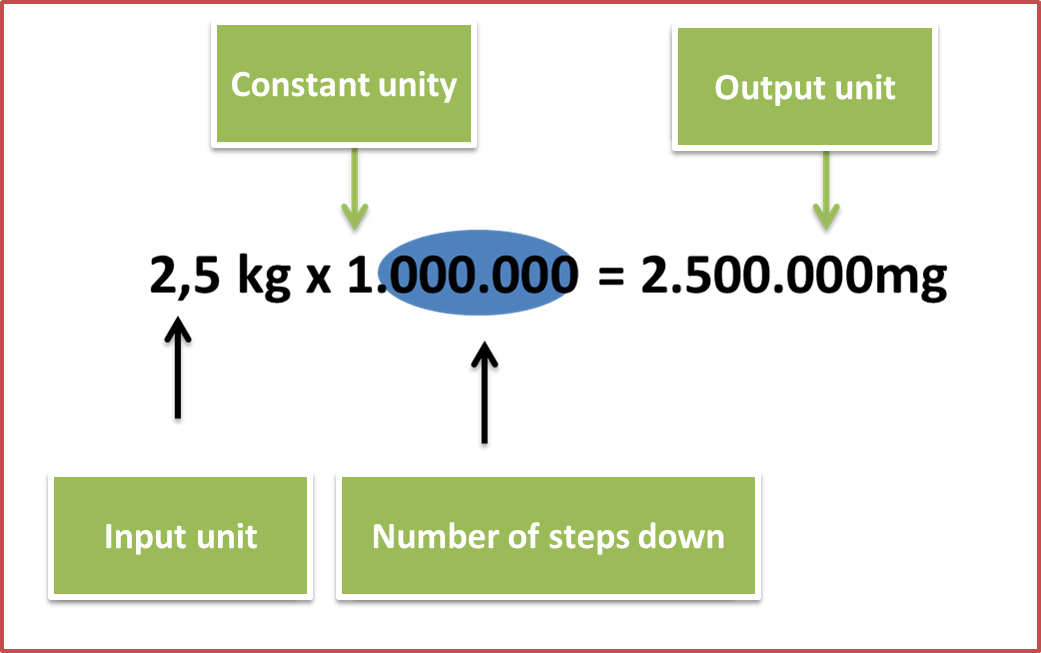
Fig. 5. Results of the proposed approach. Author: @madridbg, through Power Point 2010.
Sequence to follow:
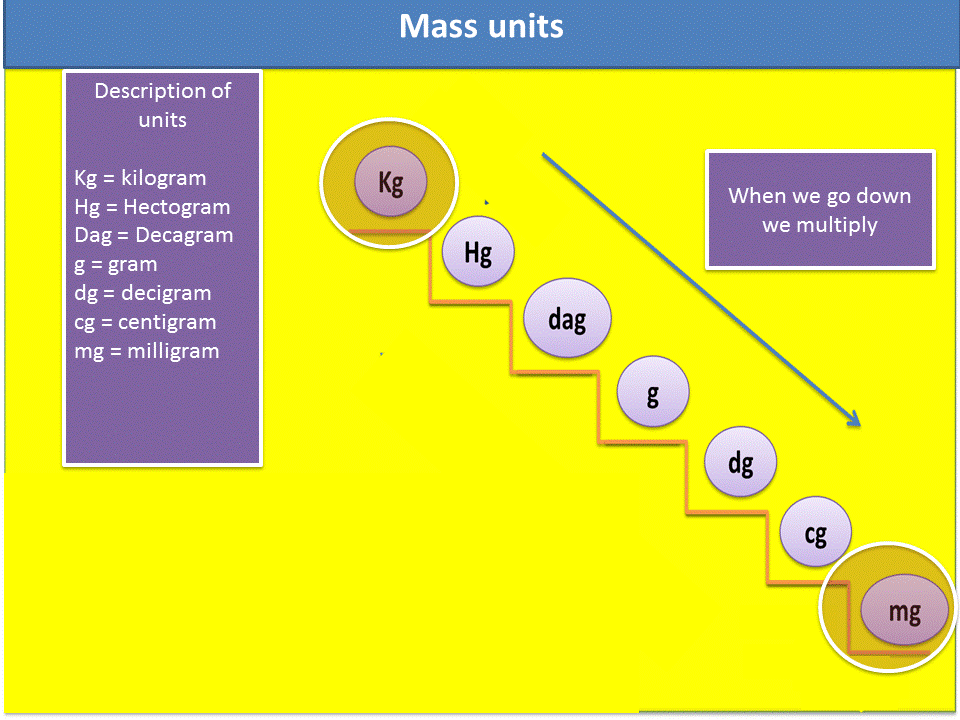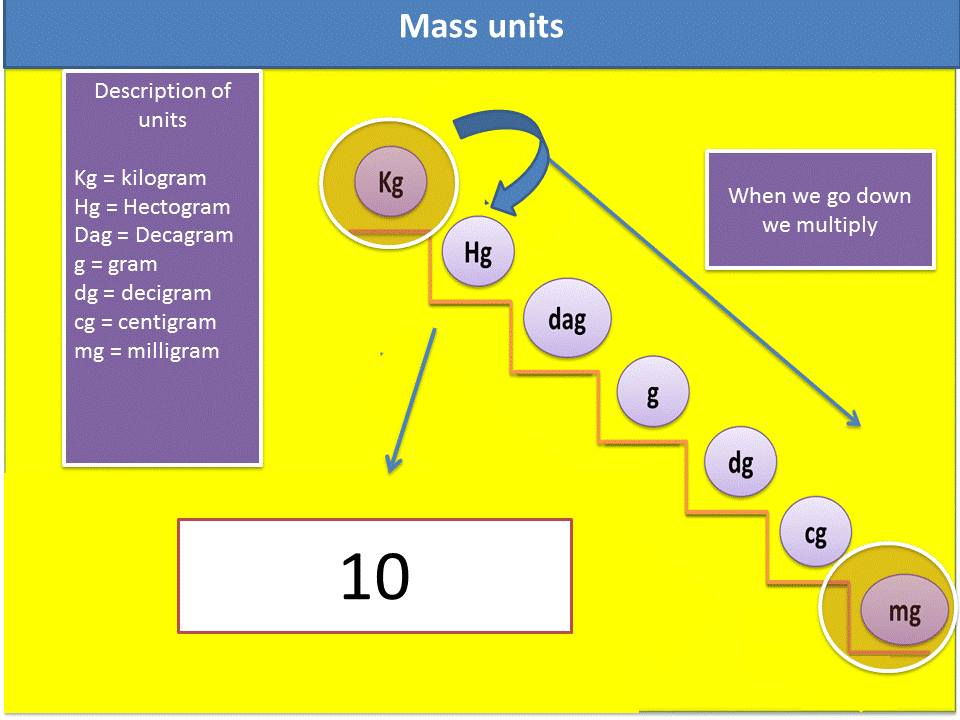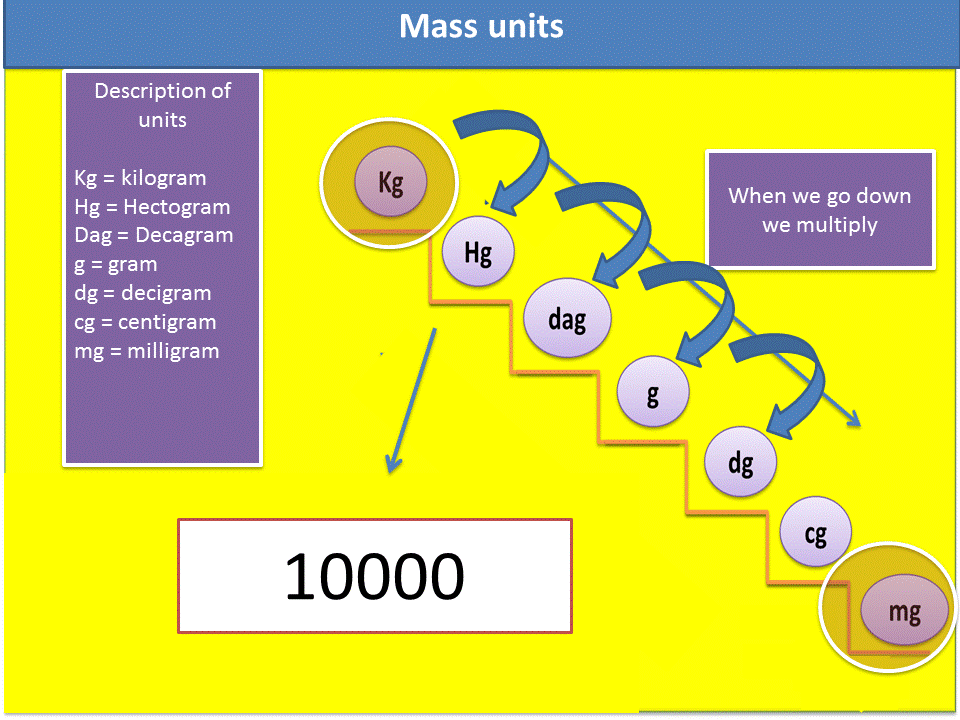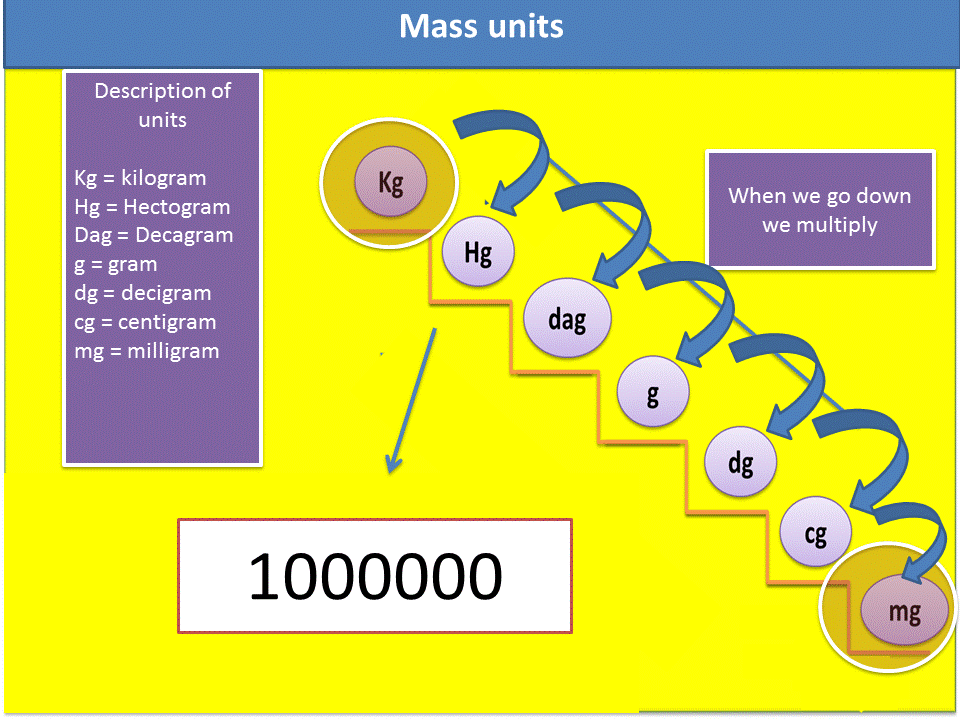
Fig. 6. Sequence to solve the exercises. Author: @madridbg, through Power Point 2010.
TOXICITY AND RISK OF CHEMICAL PRODUCTS
As mentioned in previous sections, the evolutionary process of humanity and the constant research in the area of science and technology has brought great benefits to society, but without a doubt, it has also provoked risks of which we are aware that they exist and even so we decided to assume them.
For example, we are aware that cigarette smoking kills more than 400,000 people in the United States each year and yet we still make the decision to smoke. The same happens with chemical products, understand as those substances produced in a laboratory, they can be composed by pure substances or by combinations of these, where some are innocuous for our organism and others cause fatal damages to it.
Based on the above, it is appropriate to ask ourselves, ¿How risky are the chemicals? Knowing that every day we hear in the media that the soils are contaminated, covered with pesticides and full of dangerous additives, that chemicals are responsible for so many diseases and that the drugs consumed daily are not safe.
For this reason, the need to address this issue is to make the reader understand that even our bodies are full of chemicals and that there are no cosmetics, medicines or food free of risk.
Similarly explain, that there is no marked difference between a substance produced in nature and a substance produced in a laboratory, that there are natural dangerous substances (snake venom) and harmless synthetic substances (polyethylene) and vice versa.
Therefore, on a toxicological level the risk of chemical products is measured through test animals (mice) which are treated with a high load or dose of such product and thus determine the damage they cause to the species. This process is known as acute chemical toxicity, it is reported as a value of LD50, which refers to the amount of substance per kilogram of body weight given to the animal, this dose is lethal for 50% of the test species.

Author: @madridbg, through Power Point 2010.
Although LD50 values are established in test animals, it is difficult to predict the risks that such products will cause in humans, since high doses are administered to a small animal (mice) and low doses to humans. Therefore, it is assumed that the dose is what makes the difference between the harm and benefit of the chemicals, and it is important to note that all substances are toxic to certain organisms.
As an example of the above statements, the following substances are found:
1. Arsenic trioxide, is known as a classic poison, although it is also used to treat different types of leukemia.
2. Water, a common and harmless substance, but in considerable quantities dilutes the salts in the fluids of the body, generating a condition known as hyponatremia.
3. Vitamin A, so necessary for sight, in high quantities can produce cancer.
Under the following premises, we can conclude that the benefits and risks of chemical products are framed in the decision making of people and in the dosage of such products.
CONTRIBUTIONS OF THE SUBJECT
Through the above, it becomes evident that chemistry is present in all aspects of our daily life, allowing to contextualize and relate contents such as the transformation of mass units, in such a relevant aspect as the dosage of chemical products. Likewise, it allows the reader to have a valid point of reference in the area of research and experimentation, giving the opportunity to question the reason for the observed phenomena.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Gunnar Nordberg. Metals: Chemical Properties and Toxicity Encyclopedia of Health and Safety at Work. Summary. Article: Online Access
[3] Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2002) . Bioquímica. PEARSON EDUCACIÓN, S. A., Madrid, ISBN: 978-84-832-2694-0. Materia: Bioquímica, 577. ÁREA: CIENCIAS
[4] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[5] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[6] Vargas Francisco. (1996). Prevention And Control Of Chemical Products Risk. Rev Esp Public Health. 70: 409-420. N. 4. Article: Online Access
[7] WADE, LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/BGMadrid/status/1335063644422492162
#posh twitter
https://twitter.com/BGMadrid/status/1335063644422492162?s=20
Congratulations @madridbg! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support. Using the STEMsocial app could yield even more supporti next time.