APPLIED CHEMISTRY// Brachinus crepitans, The Bombardier Beetle
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of the #Hive community, in this issue we will be talking about how wonderful chemistry tends to be, applied to nature. Therefore, we will analyze in detail the defense mechanisms used by the bombing beetles (Brachinus crepitans), which is associated to the principles of thermochemistry.
As it has been constant, my scientific publications are shared through the community of @stemsocial, who promote from their platform the contents associated with chemical, biological, mathematical interest, among others.
INTRODUCTION
Today's society has remained intact due to the different defense mechanisms and evolution that has managed to develop, likewise the biotic systems of the planet have somehow managed to stay in balance, despite the overwhelming changes that man has generated in his environment.
Currently, we can observe species that have mutated or have adapted to the environment using defense mechanisms or techniques for their survival, an example of this are the chameleons, which have managed to change the pigmentation of their skin to confuse possible predators, Similarly the butterflies Limenitis, that have managed to replicate the smell and unpleasant taste of the monarch butterflies Danaus which has allowed them to endure in fiercely competitive environments, where the survival of the fittest prevails.
The scientifically named Brachinus crepitans , has not been left behind, it has developed a surprising technique for its survival that involves chemical reactions catalyzed by enzymes with high levels of enthalpy. Concepts that we will develop throughout this publication.
Similarly, to instruct the reader in thermochemical principles we will address the different energy changes that can occur in a chemical reaction and will explain the aerosol effect as a chemical weapon that the bomber beetle has developed.
CHEMICAL REACTIONS AND THEIR ENERGY CHANGES
As mentioned in previous publications, a chemical reaction is the process that occurs when a set of reagents interact or react to form new substances (products) with different characteristics from those of the starting compounds.
Therefore, it is expected that in chemical reactions there is absorption or release of energy that in general tends to be in the form of heat, understood as the transfer of thermal energy between two bodies that are at different temperatures [2].
In this sense, in order to instruct the reader on the functioning of heat in chemical reactions, it is necessary to know some concepts associated with thermochemical , understood as the study of the variations or changes of heat in chemical processes involving reactions. [4]
When talking about thermochemistry, it is remarkable to talk about system or part of the universe that will be studied, systems can be classified as open systems (A), when there is an exchange of mass and energy in the form of heat, for example if we put to boil a pot of water we observe that water vapors come into contact with their environment.
For its part, a closed system (B), allows the transfer of heat through the walls of the container but not mass, for example a pressure cooker that does not have an exit orifice of water vapor. Last but not least we have the insulated system (C), which prevents heat and mass transfer, for example a vacuum coated vessel.

Fig 2. Type of thermochemical systems. Author: @madridbg, through Power Point 2010.
If we analyze what happened in the three systems, we can determine that the energy that is lost in the form of heat in systems A and B is gained by the environment, which represents an exothermic process, where the system has yielded heat to its surroundings, therefore chemically the total energy of the products tends to make less than the energy of the reagents.
In contrast, there is an endothermic process, where the system absorbs energy from the environment, and the temperature variation between reagents and products depends on the amount of energy absorbed.
In correspondence with the concepts studied we can establish that they are closely related to the first Law of thermodynamics, which states that energy can be converted from one form to another, but can not be created or destroyed. [4]
REACTION ENTHALPY AND HESS' LAW
In term enthalpy, it refers to the relationship that exists between the internal energy of a system with the pressure and the volume of this system, taking into account that the chemical reactions are developed at constant pressures, according to the following equation.
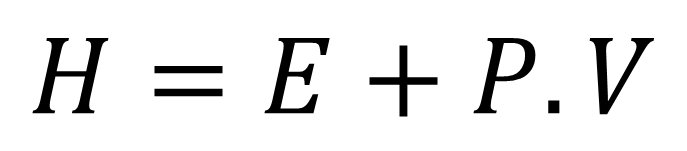
Equation 1. Representation of the enthalpy in a system. Author: @madridbg, through Power Point 2010.
Where H, represents the enthalpy, E, the internal energy of the system, P and V, the pressure and volume respectively of the system. Now, in function of instructing the reader about Hess's Law we will talk about variation of the enthalpy (ΔH) and the equation will represent it in the following way.
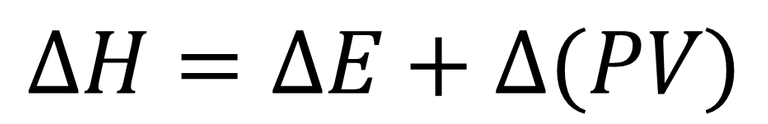
Equation 2. Representation of enthalpy variation according to Hess's law. Author: @madridbg, through Power Point 2010.
At the level of chemical reactions, the variation of the enthalpy can be negative, when the process is endothermic, that is, during the reaction energy was absorbed from the environment, on the other hand, when energy is released in the form of heat towards the environment, the enthalpy would be positive and we would be in front of an exothermic reaction.
The variation of the enthalpy in chemical reactions is represented according to the following equation.
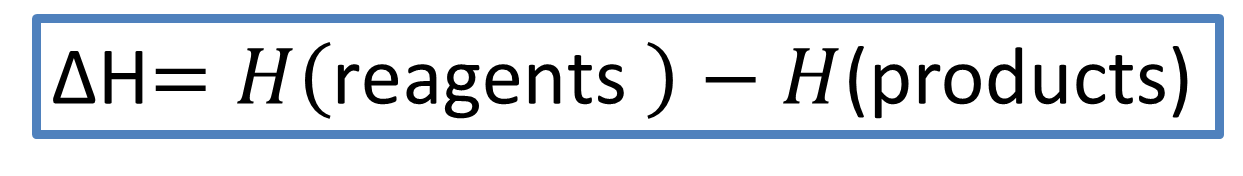
Equation 3. Chemical reactions and their relationship with enthalpy variation. Author: @madridbg, through Power Point 2010.
At this point, the chemical equations now become thermochemical equations, as they show both enthalpy changes and mass ratios. As we can see in the following equation. [5]
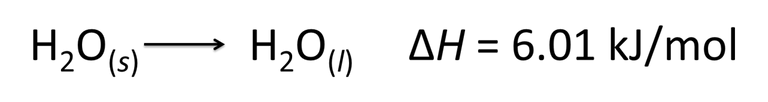
Equation 4. Examples of thermochemical equations. Author: @madridbg, through Power Point 2010.
If we analyze the equation, we can extract relevant information from the phenomenon that is occurring, therefore it is established that one mole of ice is melting, generating 1 mole of water, the variation in the enthalpy tells us that during the process there was a release of energy to the environment (exothermic reaction or change).
In reference to the Hess's law, this establishes:
"When the reagents are converted into products, the change of enthalpy is the same regardless of whether the reaction takes place in one step or in a series of steps". [2]
In other words, if during a reaction intermediates are produced, the variation of the enthalpy will be equal to the sum of all substances involved.
DEFENSE MECHANISM OF THE BOMBER BEETLE
The Bombardier Beetle uses a more active defense mechanism than those used by the Limenitis butterflies, it applies thermochemical principles that allow it to repel its predators, using a mixture of reagents, catalyzed by enzymes, is understood as a specialized biological substance that accelerates the speed of the reactions, managing to produce in its interior a great level of enthalpy, generating heat of reaction above 100°C, which then expels towards its aggressor in the form of chemical spray and audible bursts.
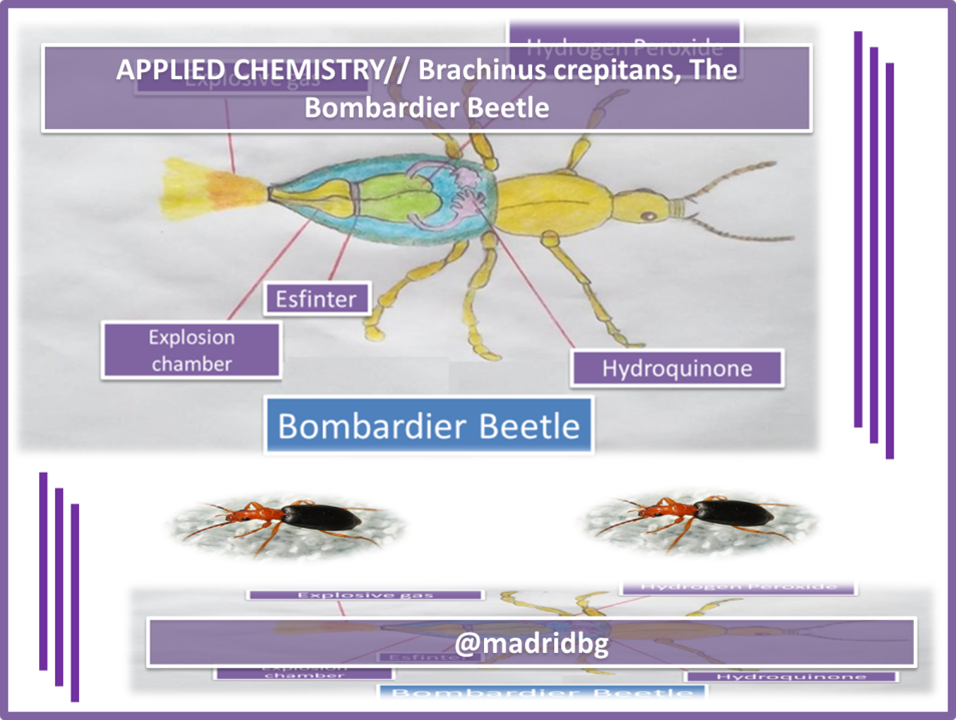
Structurally the Brachinus crepitans, has two types of glands and four compartments in its abdomen. The internal compartment, is composed of a solution of hydroquinone and hydrogen peroxide and in the external compartment are the enzymes ready for the reaction to develop, according to the following equation
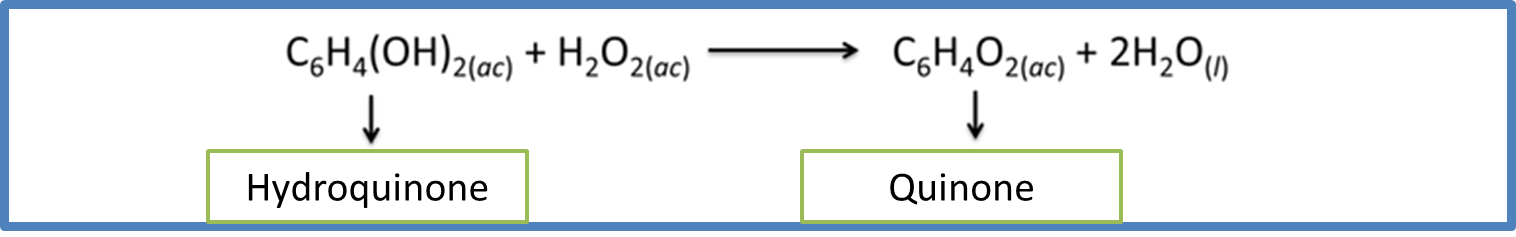
Equation 5. Representation of the thermochemical process that occurs inside the beetle. Author: @madridbg, through Power Point 2010.
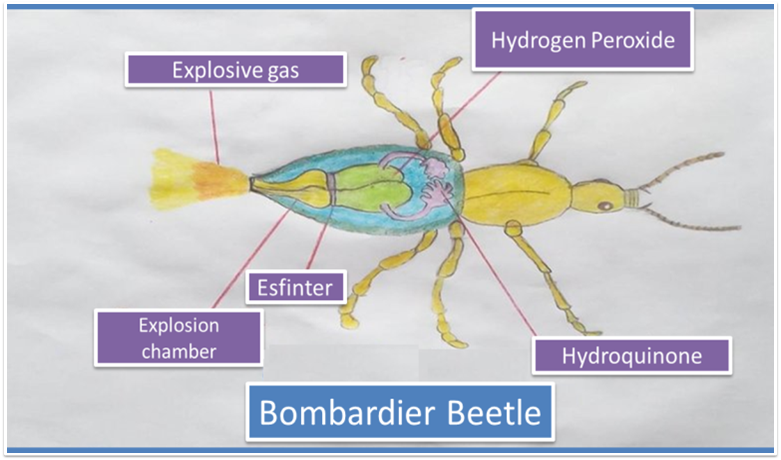
Fig 3. Structure of the Bombardier Beetle. Author: @madridbg, through Power Point 2010.
By studying the reaction of quinone formation from hydroquinone, a large amount of energy is released according to Hess's Law of 204 KJ/mol. The amount of heat generated allows the mixture to be brought into the beetle's abdomen to the boiling point (100°C) and when it feels threatened it discharges it in the form of steam. In addition, quinones act as an insect and animal repellent. In some frogs they manage to make these regurgitate (vomit), because they show their chemical potential once they have been swallowed, a detail that we can see in the following audiovisual material produced by National Geographic Spain
Video 1. Audiovisual material demonstrating the survival capabilities of the Bombardier Beetle. National Geographic España
CONTRIBUTIONS OF THE THEME
Thermochemistry is one of the concepts associated with thermodynamics that is most difficult to understand because of the complexity of its laws. Therefore, the material presented allows us to show natural phenomena and their operation through chemical foundations, which leads to an integration of knowledge from the abstract or complex to the simple and understandable for the whole society.
BIBLIOGRAPHY CONSULTED
[1] Anaya, A. (2003). Quinones and the Bombardier Beetle. Chemical ecology. Plaza and Valdés editions. ISBN: 970-722-113-5. Article: Online Access
[2] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[3] Groel, N (2006). Generation of carbon dioxide by the reaction of an acid and a base Aquariological Silver Society . Article: Online Access
[4] Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2002) . Bioquímica. PEARSON EDUCACIÓN, S. A., Madrid, ISBN: 978-84-832-2694-0. Materia: Bioquímica, 577. ÁREA: CIENCIAS
[5] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[6] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[7] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
CONSULTED WEBSITES
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/BGMadrid/status/1343298145938243584
#posh twitter:
https://twitter.com/BGMadrid/status/1343298145938243584?s=20
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.