Chemistry of water -Part 2-
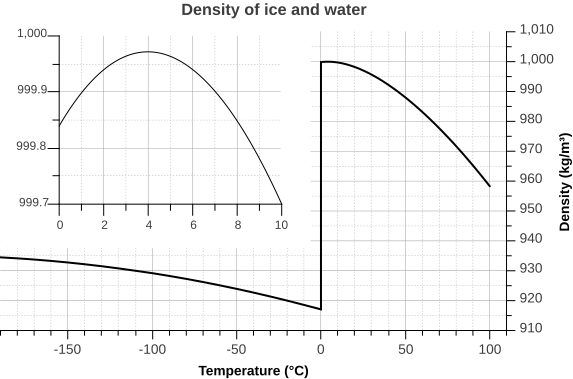
Since water is the densest at +4 °C, it expands like other liquids when heated above this point, but it also does so when its temperature drops below this point. In contrast to most compounds, water also expands when it freezes, which is why ice is lighter than water.
![]
( )
)
Density of ice and water as a function of temperature
Because of the strong electronegativity of the oxygen atom, the water molecule is polar, and this polarity leads to a rise in its dielectric constant, which makes water play a very important role as a solvant for polar and ionic compounds. In addition, this results in the formation of hydrogen bonds between water molecules, which are responsible for the molecules interconnection and for the fact that water is a liquid rather than steam under normal heat and pressure conditions.
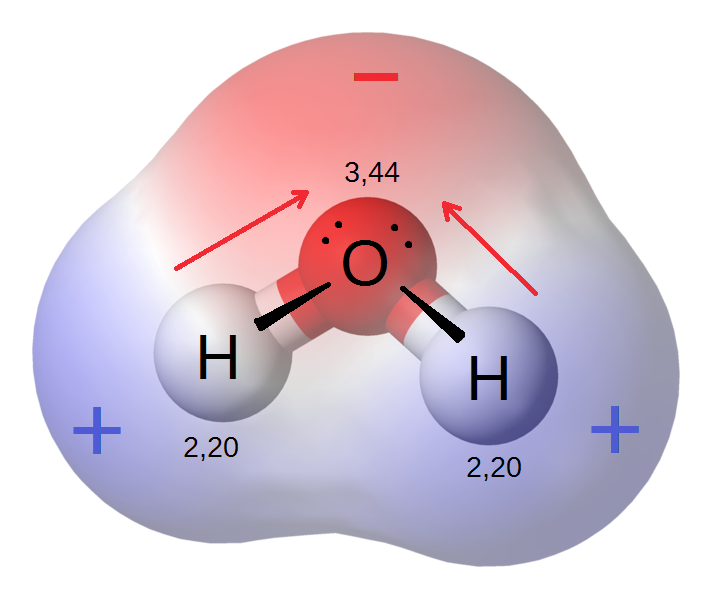
Permanent dipoles in the water molecule
The water molecule's positive and negative charge centers of gravity are separate, and the water molecule's total dipole moment is 1.85, the unshared pair of electrons most likely adds to the significance of this dipole moment.
The water molecule forms hydrates with the widest range of ions with ease. The hydroxonium (or oxonium) ion H3O+ is one of these hydration reactions' particularly significant products. This ion is found in any aqueous solution that has protons-detaching bodies. Although the affinity of the water molecule for the proton is relatively strong, we only know its value in the gaseous state, which is 169 kcal/mol, the affinity for the proton of other molecules is even stronger; thus, for ammonia, it is 206 kcal/mol, which explains how the ammonia molecule is able to take the protons from the water molecules by producing ammonium ions.
Bibliographic references:
[General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
Kada, Imene- Double ionisation de la molécule d’eau par impact d’électrons- Phd thesis
[Book- Chimie moderne- L.Nikolaiv]
Berend Ensing; geboren te Delfzijl- Chemistry in Water. Phd Thesis. University of Amsterdam- 2003
[Smith, Jared D.; Christopher D. Cappa; Kevin R. Wilson; Ronald C. Cohen; Phillip L. Geissler; Richard J. Saykally (2005). "Unified description of temperature-dependent hydrogen bond rearrangements in liquid water" (PDF). Proc. Natl. Acad. Sci. USA. 102 (40): 14171–14174. Bibcode:2005PNAS..10214171S. doi:10.1073/pnas.0506899102. PMC 1242322. PMID 16179387.]
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
[Livre- Chimie génerale- R.Ouahes]
Es interesante tu artículo, mantiene una secuencia explicativa que ilustra nuestro conocimiento. El hablar del agua desde el punto de vista químico, es un trabajo de investigación arduo, una labor que nos permita exponer nuestras conclusiones, lo cual es muy importante.
Gracias @benainouna por tu aporte a esta comunidad de @stemsocial
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Congratulations @benainouna! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next payout target is 500 HP.
The unit is Hive Power equivalent because post and comment rewards can be split into HP and HBD
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!
Dear @benainouna,
May I ask you to review and support the Dev Marketing Proposal (https://peakd.com/me/proposals/232) we presented on Conference Day 1 at HiveFest?
The campaign aims to onboard new application developers to grow our ecosystem. If you missed the presentation, you can watch it on YouTube.
You cast your vote for the proposal on Peakd, Ecency, Hive.blog or using HiveSigner.
Thank you!