The Science of Freezing Moving Water
This is another epic video by the famous The Action Lab YouTube channel. This time he goes over the science of freezing moving water, and freezing in general.
Although the freezing temperature of water is 0°C, it doesn't mean water has to freeze but rather it can freeze at this temperature. Beyond just the temperature, water needs to form a crystalline structure in order to freeze. This process requires a starting point called a nucleation site. This is illustrated by placing a bottle of water inside the freezer for several hours: the water is well below freezing temperature but it is still in liquid form, this is called supercooled water. To freeze the bottle of water, you can strike the bottle hard or just pour it ontop of ice, in both cases a nucleation site is created and the water begins to rapidly freeze!

Preventing a nucleation site from forming is one way of preventing water from freezing, but another way is to prevent the crystalline structure from forming at all. This can be done by constant motion of the water, much like a flowing river in arctic conditions even when the land is covered with snow. In the video, James J. Orgill (the guy that runs The Action Lab) demonstrates this buy placing a pump inside a bucket of water that constantly pushes air to circulate the water. Then placing liquid nitrogen to try to freeze the water, it takes quite a bit of effort but the pump froze before the water does.

Another experiment he tries is spinning the water via a magnetic stirrer, and then placing it inside a cup of liquid nitrogen. After a while the water eventually freezes while still in its whirlpool vortex formation! Repeating the experiment but with pouring the liquid nitrogen, the freezing is shown slowly from the top to the bottom. The color of the ice is very white as it has lots of dissolved air that didn't have enough time to coalesce and rise upwards. This results in a lot of light scattered in all directions, hence the white appearance.

Even though the water is moving rapidly, it can still freeze at cold enough temperatures because of a condition called a "no slip condition". This is often the case at the boundary of a moving fluid and the surrounding wall. In this case, the water that makes contact with the cup is not moving fast or at all relative to the rest of the fluid. That is, it is not "slipping".
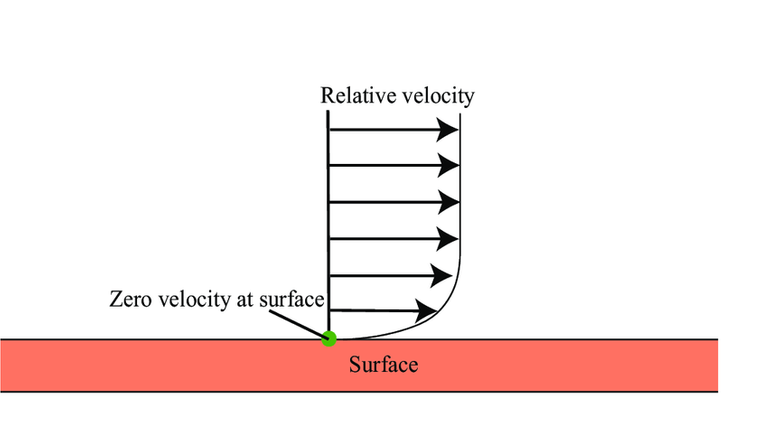
This is some very interesting fluid and temperature dynamics!
This is so educative, thanks for sharing.