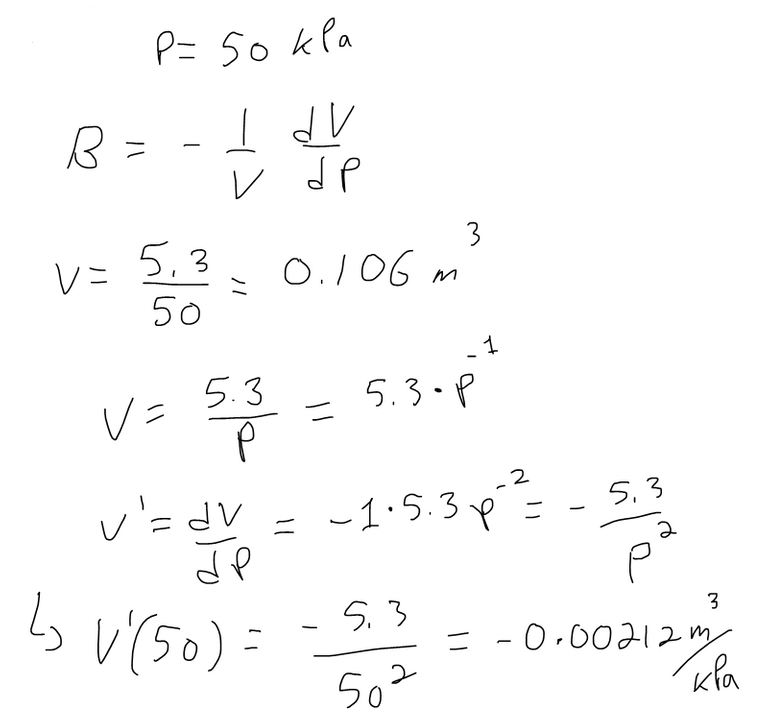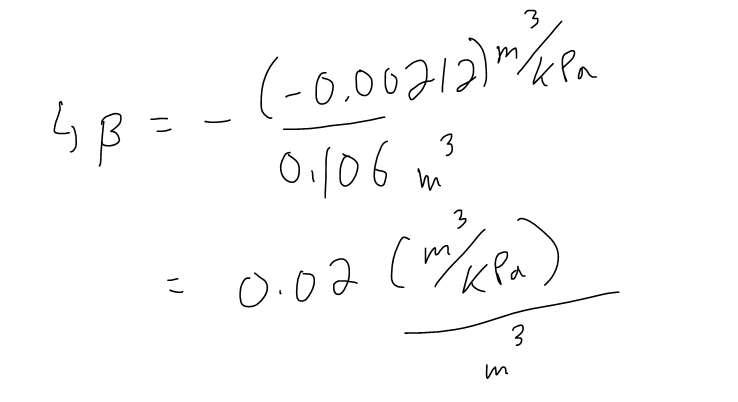Derivatives Application: Compressibility
In this video I go over another derivatives application and show how isothermal compressibility can be described using the derivative as a rate of change of the volume with increasing applied pressure. I also go over an example in determining the compressibility of air at 25 degrees Celsius.
Watch video on:
- BitChute: https://www.bitchute.com/video/MYEc4x9YtteW/
- 3Speak:
- DTube: https://d.tube/#!/v/mes/tx6xly4casr
- YouTube: https://youtu.be/NoXz7eIRELk
Download video notes: https://1drv.ms/b/s!As32ynv0LoaIiKtVszaCgz60JI8tZg?e=snqrAM
View Video Notes Below!
Download these notes: Link is in video description.
View these notes as an article: https://peakd.com/@mes
Subscribe via email: http://mes.fm/subscribe
Donate! :) https://mes.fm/donateReuse of my videos:
- Feel free to make use of / re-upload / monetize my videos as long as you provide a link to the original video.
Fight back against censorship:
- Bookmark sites/channels/accounts and check periodically
- Remember to always archive website pages in case they get deleted/changed.
Join my private Discord chat room: https://mes.fm/chatroom
Check out my Reddit and Voat math forums:
Buy "Where Did The Towers Go?" by Dr. Judy Wood: https://mes.fm/judywoodbook
Follow along my epic video series:
- #MESScience: https://mes.fm/science-playlist
- #MESExperiments: https://peakd.com/mesexperiments/@mes/list
- #AntiGravity: https://peakd.com/antigravity/@mes/series
-- See Part 6 for my Self Appointed PhD and #MESDuality breakthrough concept!- #FreeEnergy: https://mes.fm/freeenergy-playlist
NOTE #1: If you don't have time to watch this whole video:
- Skip to the end for Summary and Conclusions (if available)
- Play this video at a faster speed.
-- TOP SECRET LIFE HACK: Your brain gets used to faster speed. (#Try2xSpeed)
-- Try 4X+ speed by browser extensions or modifying source code.
-- Browser extension recommendation: https://mes.fm/videospeed-extension
-- See my tutorial to learn more: https://peakd.com/video/@mes/play-videos-at-faster-or-slower-speeds-on-any-website- Download and read video notes.
- Read notes on the Hive blockchain #Hive
- Watch the video in parts.
NOTE #2: If video volume is too low at any part of the video:
- Download this browser extension recommendation: https://mes.fm/volume-extension
Derivatives Application: Compressibility
Compressibility
One of the quantities of interest in thermodynamics is compressibility. If a given substance is kept at a constant temperature, then its volume V depends on its pressure P.

We can consider the rate of change of volume with respect to pressure – namely, the derivative dV/dP. As P increases, V decreases, so dV/dP < 0.
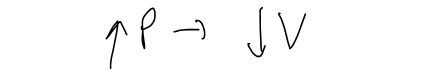
The compressibility is defined by introducing a minus sign and dividing this derivative by the volume V:
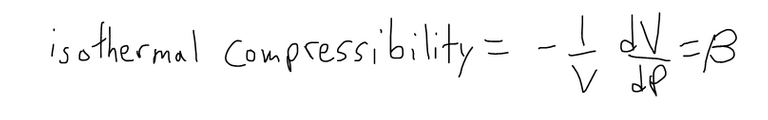
Thus, β measures how fast, per unit volume, the volume of a substance decreases as the pressure on it increases at constant temperature.
Example
If the volume V (in m3) of a sample of air at 25°C was found to be related to the pressure P (in kPa) by the equation:
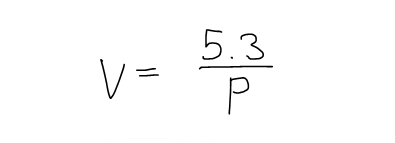
Determine the Compressibility of the air at a pressure of 50 kPa.