New method, based on an old technique, to produce slow release fertilizers
The chemical fertilizer industry has an important challenge to face, to develop materials that release nutrients in a controlled way into the soil and also avoid environmental pollution. These materials, called slow-release fertilizers, improve the utilization of nutrients by plants, since they are available for a longer period of time, which avoids losses of their components and their dispersion in the environment. That is why an international team optimized an old method to produce a solid compound that slowly releases nitrogen and calcium, two very important elements in soil fertilization.

Nitrogen fertilization is essential in agriculture. Source: Wikipedia.org.
What is a Slow Release Fertilizer?
Slow-release fertilizers are those that allow the plants to have the elements that compose them in different ways, one is by delaying the availability of these elements when they are taken up by the plants, so that they can be used after they are applied, another way is to provide a mechanism that allows the availability of these nutrients for a long period of time.
Regarding the second way, prolonging the time of availability of nutrients can be achieved in different ways, some of the most studied have been by protecting the material with semi-permeable coatings, by occlusion or encapsulation in polymers, and even some chemical mechanisms such as slow hydrolysis of soluble compounds.

A slow release fertilizer is available to the plant for a prolonged period of time. Source: Wikimedia.org.
An advanced milling technique
Until now, to produce the common nitrogen fertilizers, Urea and ammonium nitrate, the Haber-Bosh process is used, which is a process of ammonia synthesis through the reaction of nitrogen and hydrogen, under conditions of high pressure (200 atm) and high temperature (450-500 ºC), and in the presence of a catalyst, commonly iron compounds.
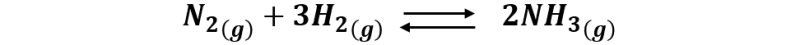
But this method consumes a lot of energy, and when pure urea is used as fertilizer, its decomposition quickly releases large amounts of ammonia.
But a team of scientists at the Ruder-Boskovic Institute in Zagreb have developed an alternative and more environmentally friendly method of producing a nitrogen fertilizer, using an ancient technique, mechanochemistry, which basically involves using mechanical force to synthesize new materials and compounds.
And this team of researchers relied on the grinding of two ingredients, urea and gypsum, to produce a solid compound that slowly releases nitrogen and calcium, two fundamental elements for soil fertilization, into the soil.
Specifically, the research group explored the synthesis of a calcium urea sulfate ([Ca(urea)4]SO4) cocrystal using thermally controlled mechanochemical methods, first conducting small-scale experiments in a stirring mill, monitoring the process by in situ Raman spectroscopy, and by X-ray diffraction using DESY's PETRA III X-ray source to investigate what the resulting powder consists of. The PETRA III beamline is a facility in which various types of chemical reactions can be analyzed using X-rays from a synchrotron, allowing the evolution of the mixture to be determined by applying radiation directly to the grinding vessel. The results of this research have recently been published in the journal Green Chemistry.
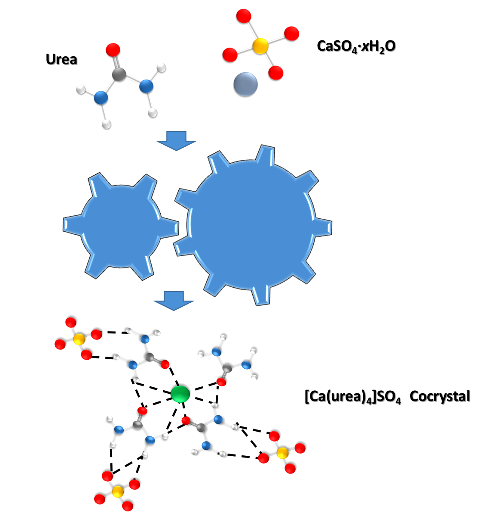
A mechanochemical method for forming urea and calcium sulfate cocrystals. Source: Image elaborated in powerpoint.
The grinding method made it possible to obtain a cocrystal, a solid whose crystalline structure is composed of two substances stabilized by weak intermolecular interactions that form repeating patterns. In this case, milling allowed the calcium sulfate of gypsum and urea to bind together. And according to the results, this cocrystal has a solubility in aqueous solution almost 20 times lower compared to Urea, and in addition, the reactive nitrogen emissions of this material, expressed as an amount of ammonia, were shown to be slow and progressive over 90 days, pure urea took between 1 and 2 weeks to reach the same level of emissions.
According to the researchers, pure urea forms a crystal that falls apart easily, releasing the nitrogen contained very quickly; but with calcium sulfate, through the milling process, a cocrystal is obtained that is strong enough to allow a slow release of nitrogen and calcium.
On the other hand, among the advantages of this process is that the mechanochemical process does not generate any hazardous by-products, and although the process has yet to be tested on an industrial scale and the fertilizer has yet to be tested in the field, the results are encouraging enough to continue to scale up and conduct field trials.
Hopefully, the field trials will achieve good results and a method can be obtained to reduce dependence on the traditional process, whose products release large amounts of nitrogen into the environment, while slow-release fertilizers avoid high concentrations of ions in the soil due to the rapid solubility of the product, which also reduces the impact of these products on the environment by reducing losses of ammonium ions and emissions of N2O gases into the atmosphere.
Thanks for coming by to read friends, I hope you liked the information. See you next time.
References
Mayra, González (2005). Obtención de un fertilizante de liberación lenta y controlada enriquecido con diferentes plantas Marinas. Revista Cubana de Química, Vol. XVII, No 3.
Ivana Brekalo, Valentina Martinez, Bahar Karadeniz, Donata Drapanauskaite, Hein Vriesema,Robert Stenekes, Martin Etter, Igor Dejanović, Jonas Baltrusaitis and Krunoslav Užarević; (2022). Scale-Up of Agrochemical Urea-Gypsum Cocrystal Synthesis Using Thermally Controlled Mechanochemistry. Sustainable Chemistry & Engineering“.
!1UP
You have received a 1UP from @luizeba!
@stem-curator, @vyb-curator, @pob-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
Thanks for your support!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
thanks for the support friends!
This is an interesting blog. I have a very basic question. Do you know under which circumstances you would use a slow-release fertiliser, instead of a fast one. May I have your opinion on this? Thanks in advance.
Cheers!
Hello friend, what a pity to reply so late to this comment, I don't know how I let it pass. I could not mention some specific circumstances, I only know that a large part of the nitrogen that is added to the soil by fertilizing is lost because ammonia is very soluble and also volatile, perhaps in regions with frequent rains fertilizing the soil in this way represents great losses, being preferable to use slow-release fertilizers.
This is fine, don't worry. Better a late answer than no answer at all! Thanks for coming back to me!
Cheers!