What’s about Acetone that ruins nail polish?
About a year or two ago during my internship in a Public Analyst laboratory, I experienced something that blew my mind.
We were performing a particular weight identification analysis where acetone was put in a crucible to get rid of all moisture that will alter the weight of the crucible(being that acetone evaporates when exposed to air). In the process of handling the acetone to put in the crucible, some spilt on my nail polished fingernails and it got ruined and that birthed the curiosity question
WHY?
Acetone is a component of most nail polish removers(before the time of the incident I had no idea).
Nail polish contains certain components that make up its composition;
•Film forming agents e.g Nitrocellulose
•Resin e.g castor oil, mixes of glycerol, acetic acids, etc.
•Plasticizers e.g camphor, dibutyl phthalate.
•Solvents e.g butyl acetate, ethyl acetate, etc.
•Colouring agents e.g pigments.
The Film-forming agents; Nitrocellulose, which is also the major component of nail polish forms a hard coating or film on the exposed surface of the finger or toenail serving as a covering but in itself, sticks poorly to nails cause it dries quickly, becomes brittle and breaks off.
Resins alongside plasticizers are the components that improve the flexibility, pliability and resistance to moisture and soap.
Resins help the film to stick to the fingernails after the solvent evaporates, also helping to give that glossy look and texture after application.
Plasticizers specifically improve the durability of nail polish.
Solvents are used to hold all the components (film-forming agents, colouring agents, resins, plasticizers, etc.) together, we could as well say that without the use of solvents, each component will be incapable of mixing. After application of the nail polish to the fingernails, the solvent evaporates so it is advisable to keep the lid of the nail polish shut when not in use.
Colouring agents gives each nail polish its distinctive colour and aesthetic value, in ancient times, soluble dyes were used to give colour but in modern days most nail polishes contain pigments of one kind or the other. For example, inorganic pigments like Chromium oxide get green colour, cobalt for blue and organic pigments like carotene that give a yellow/orange colour.
The presence of immiscible components in nail polish makes the nail polish a suspension product i.e products with components that are made up of minute particles not able to completely dissolve, and can only be held by a solvent for a relatively short period. That’s why shaking a bottle of nail polish before use help restore these particles to their suspension state reason being that some may settle at the bottom. A suspension can also be said to be a heterogeneous mixture in which solute particles are spread throughout in a solvent without being entirely dissolved in it.
The main component of nail polish; Nitrocellulose, is our point of concern and Acetone being the major component of most nail polish removers dissolves Nitrocellulose because it is an excellent solvent for Nitrocellulose.
THE CHEMISTRY OF NITROCELLULOSE
The synthesis of production of Nitrocellulose is possible via the use of a Nitration Reaction.
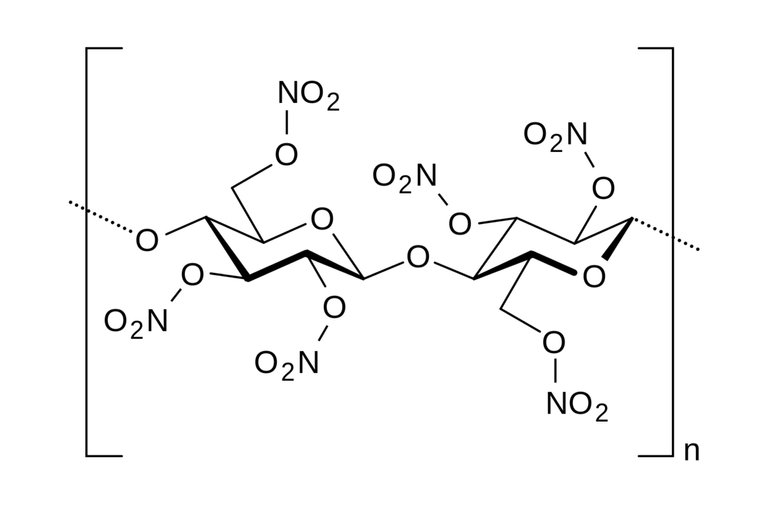
Skeletal structure of nitrocellulose
Nitrocellulose also known as cellulose nitrate is a white cotton-like substance it is produced by treating cellulose with a mixture of nitric acids and sulphuric acid(Cellulose is a polymer gotten from wood pulp or little fibres that stick to cotton seeds and it has the chemical formula; (C6H10O5)n).
The consequence of this reaction changes the hydroxyl groups(-OH) present in the cellulose to nitro groups(-NO2).
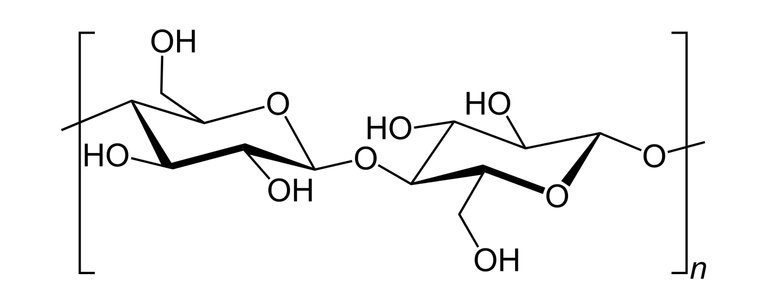
Skeletal structure of cellulose

Image of Cotton fibre, a source for cellulose
Nitrocellulose is a nitrate ester, In the structure of the cellulose compound, three hydroxyl(-OH) ions are present, giving chance for the substitution with Nitro group(-NO2) in the three sites of reaction or all at once. When one molecule of hydroxyl ion is substituted, it is regarded as mononitrocellulose, with two molecules as dinitrocellulose then the three molecules as trinitrocellulose and this is possible by increasing the concentration of the nitrating agents in each case as their names imply; mono- increased by one, di- increased by two and tri- increased by three.
The chemical formula are shown below;
(C6H9(NO2)O5)n(mononitrocellulose)
(C6H8(NO2)2O5)n (dinitocellulose)
(C6H7(NO2)3O5)n (trinitrocellulose)
These distinct substitutions gives varying strength and functions to each of these Nitrocellulose molecule; dinitrocellulose is useful in making most lacquers and varnishes while trinitrocellulose is highly flammable and used in the making of explosives, rock propellant, flash paper, etc.
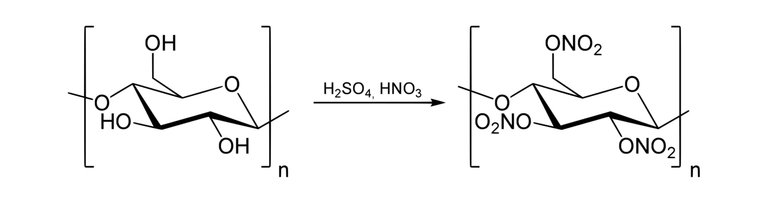
Synthesis of nitrocellulose from cellulose
Nitrocellulose also regarded as gun cotton and a major ingredient in smokeless gun powder decomposes violently and explosively.
At a time in the early twentieth century, nitrocellulose was discovered to be of excellent use in the coating and paint industry. Nitrocellulose lacquer was also introduced for it’s spectacular use as a finish on musical instruments like guitars, violin, saxophones, clarinets, etc.
THE CHEMISTRY OF ACETONE
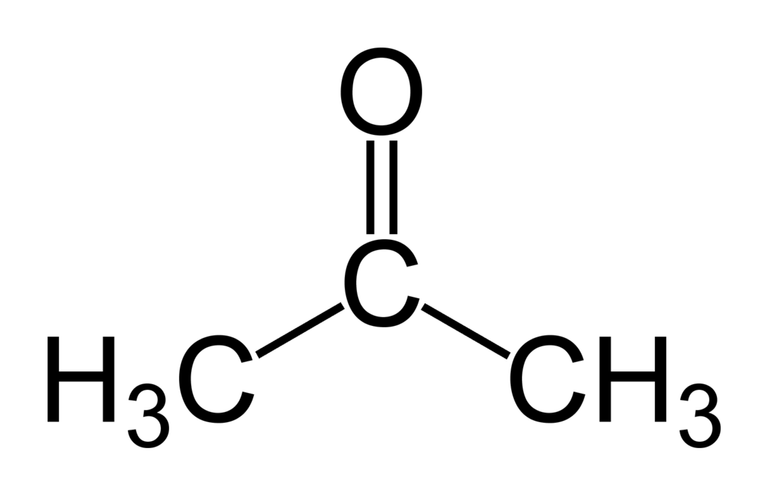
Acetone is a colourless aromatic and volatile liquid classified under the organic compounds; Ketones, having a chemical formula of (CH₃)₂CO.
Acetone is known as a polar-aprotic solvent with the ability to dissolve both polar and non-polar compounds due to the presence of two different active sites; It’s C=O bond is polar making it possible to be soluble/miscible in water(this characteristic makes it one of the few organic compounds capable of doing such) while its methyl(CH3) group is nonpolar making it an active site to interact and react with non-polar compounds/solutes. It is also capable of dissolving many resins. It has a dipole moment of 2.88D which is way higher than that of water which is 1.85D and it also has a dielectric constant of 21, making up for an excellent solvent used in many industrial purposes involving non-polar, organic and inorganic materials, and also because of it’s ability to be easily removed when no longer in use via evaporation(acetone has weak intermolecular forces due to its high vapour pressure and absence of hydrogen bonding).
THE CHEMISTRY BETWEEN ACETONE AND NITROCELLULOSE
Nitrocellulose is a nonpolar compound compared to it’s counterpart; cellulose which is polar, one of the reasons it cannot dissolve in water cause water is a polar solvent.
Acetone being a polar aprotic solvent has in itself the ability to dissolve nonpolar compounds; Nitrocellulose being no exception. Unlike the solvents used to make nail polish that keeps it’s components together, Acetone instead tears it apart, Acetone breaks the suspension and makes this components separate, it then directly attacks nitrocellulose breaking its intermolecular forces and therefore(since both acetone and nitrocellulose are volatile) evaporate when exposed to air leaving behind little or no traces of nitrocellulose, the colouring agents, and traces of the other components. Meanwhile, the solvents used in the preparation of nail polish must have evaporated on exposure to air before the introduction of acetone, other components like fat-based resin and plasticizer are also equally dissolved by acetone.
With all said and done, you now know better than to carelessly handle acetone with those beautifully polished nails🤗.
Thanks for reading, please do well to engage me in the comment session.
REFERENCES



This is a one-time notice from SCHOOL OF MINNOWS, a free value added service on hive.
Getting started on hive can be super hard on these social platforms 😪 but luckily there is some communities that help support the little guy 😊, you might like school of minnows, we join forces with lots of other small accounts to help each other grow!
Finally a good curation trail that helps its users achieve rapid growth, its fun on a bun! check it out. https://hive.blog/schoolofminnows/@schoolofminnows/someeznz9em3ap
Great info shared! That's what I love about hive, we learn all kinds of stuff!
I'm honoured, thanks for reading🤗
Congratulations @dancinglockers! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 50 replies.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!
Knew about this but not as detailed as your write up is.
You went really went deep to explain the chemistry behind the action.
I am aware isopropyl alcohol also does similar thing but not as strong as acetone.
Great one I must say.
Kudos
Well written. Thank you for sharing. :)
!1UP
You have received a 1UP from @thecuriousfool!
@stem-curator, @neoxag-curator, @pal-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
Thanks for sharing this very detailed post, in particular about how acetone functions at the chemistry level. Acetone is a very useful product, even for me who has nails that are always short and un-decorated. I indeed use it as a paint thinner (I think that this is the proper English word) at home and also as a remover for many things (like glue).
Cheers!
Thanks for reading, I am honoured.
You can say that acetone is a superhero🤭 cause of its diverse use and great importance
You are very welcome!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Wow, this was quite an interesting read! I had no idea nail polish had such a complex composition. I tend not to paint my nails very often, because I work with my hands a lot, I am lucky if it lasts a day or two before some of it has chipped off or gotten damaged somehow. 😅
Still a very interesting read all round!
That happens to me also, working with food and all, I'm glad you found the write up insightful, thanks for reading 🤗