THE CHEMICALS OF LIFE: Steroids in Athletics and the Importance of Protein to Living Organisms.
Anabolic steroids are synthetic compounds that are similar in structure to testosterone, the male sex hormone. They increase anabolic (building-up) reactions, which enhance muscle size. Athletes, notably weightlifters, began injecting testosterone in the 1950s to gain extra strength. When steroids were banned, athletes looked for other natural substances that would be undetectable in blood tests. Human Growth hormone (HGH, or somatotropin) and also erythropoietin are other illegal but potentially performance-enhancing substances. HGH increases muscle size in adults and erythropoietin increases the oxygen-carrying capacity of the blood.

HOW ANABOLIC STEROIDS WORK
Steroid hormones are lipid soluble and so can pass through the plasma membrane. Studies have shown that anabolic steroids pass into the nucleus, where they force the cell to ‘read’ the genes that code for the muscle proteins actin and myosin. As well as increasing muscle size, athletes can train for longer, and the greater aggression that they tend to develop may also give them an added competitive edge.

The use of anabolic steroids is unfair and dangerous. To achieve a muscle-enhancing effect, athletes need to inject 10 to 40 times the normal dose, and this can have irreversible side effects. Symptoms of steroid abuse include acne, impotence, sterility, diabetes, heart disease and even liver cancer. Studies of male steroid abusers have shown that their testes may decrease in size by as much as 40 per cent. Their sperm count is correspondingly lowered. Female athletes who use steroids tend to have fewer periods and can develop masculine features such as excessive body hair and a deeper voice.
PROTEINS
Proteins play a central role in the structure and metabolism of all living organisms. Protein molecules have a huge variety of shapes and sizes (the structure of carbohydrates and lipids is relatively limited by comparison). This versatility of shape is the key to the role of proteins in the cell.
THE IMPORTANCE OF PROTEINS TO LIVING ORGANISMS
We can appreciate the extent to which organisms use proteins by looking at their distribution in the human body. Tubulin forms microfilaments which make up the cytoskeleton of cells. Albumen is a blood plasma protein essential for normal circulation. Antibodies have a central role in defence against disease. Enzymes are globular proteins which control metabolism. Some hormones such as insulin, are proteins. Actin and myosin filaments produce movement, e.g in muscles. Membrane proteins are vital to the functioning of the plasma membrane, e.g, forming pores or acting as carrier molecules in active transport. Collagen gives strength to connective tissues.
Consider some of the jobs that proteins need to do:
- Each cellular metabolic reaction must be catalysed by a different enzyme.
- Each substance that passes across a cell membrane requires a different carrier molecule.
- A different antibody is needed to combat the chemicals produced by the many (and also constantly changing) disease-causing organisms such as bacteria and viruses.
Enzymes, carrier molecules and antibodies are all proteins that are ‘tailor-made’ to fulfill all these requirements.
THE STRUCTURE OF PROTEINS
Proteins are large and complex molecules. If a water molecule (relative molecular mass = 18) was the size of a brick, proteins would be whole buildings. In addition to the elements carbon, hydrogen and oxygen, proteins always contain nitrogen and sometimes sulphur. The building blocks of proteins are called amino acids. The figure below shows the basic structure of an amino acid. As the name suggests, all amino acids contain an amino group (-NH2) And a carboxylic acid group (-COOH). Both of these groups are attached to a central carbon atom, known as the α-carbon. The ‘backbone’ of an amino acid is made up from the three atoms C-C-N. The R group varies between different amino acids. There are up to 20 amino acids that make up all of the proteins in the human body, five of which are alanine, phenylalanine, cysteine, leucine and glutamic acid.
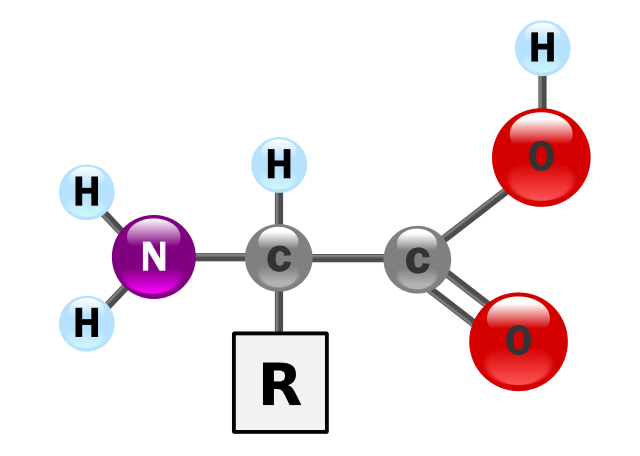
The structure of an alpha amino acid in its un-ionized form. YassineMrabet, Public Domain
Amino acids in solution
Amino acids are readily soluble in water. When in solution they can resist a change in pH by mopping up or releasing both hydrogen ions (H+) an hydroxyl ions (OH-), acting as buffers, that is, regulators of pH. In whole proteins, the buffering effect is largely due to the R groups.
Peptides, polypeptides or proteins?
It is easy to be confused about the precise meaning of the terms peptide, polypeptide and protein; there isn’t a universally accepted rule to follow. When amino acids are assembled into a short chain a peptide is formed. Longer chains are known as polypeptides. The term ‘protein’ is reserved for the finished, functional molecule. Some proteins consist of one polypeptide, others consist of two or more than two. Haemoglobin, for example, contains four polypeptides.
HOW AMINO ACIDS FORM PROTEINS
Amino acids join together in long chains to form proteins by means of peptide bonds. Two amino acids join to form a dipeptide. This is an example of a condensation reaction. All proteins are complex molecules and biochemists look at their structure at four different levels: primary, secondary, tertiary and quaternary.
Primary structure
The primary structure of a protein refers to the sequence, or order, of amino acids in that protein. A simple primary structure of a tiny protein could be shown as:
Alanine-histidine-phenylalanine-glutamine-cysteine
Real proteins usually consist of a lot more than five amino acids. The hormone insulin, for example, a relatively small protein, has 51 amino acids. The code for the primary structure of any protein is contained in the gene or genes that code for that protein. This code determines the precise order in which amino acids are assembled. This order then dictates the way they will twist and turn to produce the precise three-dimensional shape that allows the protein to carry out its specific function in the body. The first level of three-dimensional twisting is described as the secondary structure of the protein.
Secondary structure
When combinations of amino acids join together in a chain they tend to fold into particular shapes and patterns (such as spirals) in some places. These shapes form because the amino acids twist around to achieve the most stable arrangement of hydrogen bonds. The secondary structure of the protein refers to the patterns contained within the amino acid chain. Such patterns exist in different places in different proteins, producing an almost infinite variety of molecular shapes.
The main types of secondary structure in proteins are:
- The α-helix, a spiral, is the most common type of secondary structure. Amino acid residues in the spiral twist on their axis, each residue forming a hydrogen bond with another residue four units along. These hydrogen bonds stabilize the α-helix.
- The β-pleated sheet, a flat structure that consists of two or more amino acid chains running parallel to each other, linked by hydrogen bonds . The secondary structure of a protein depends on its amino acid sequence: some amino acids (or combinations of amino acids) tend to produce α-helices, others usually make β-sheets. A few amino acids tend to produce a sharp bend in the chain, a vital function that allows the chain to fold back on itself.
In the secondary structures within the enzyme lysozyme: the α-helices and β-pleated sheets are clearly visible. The α-helices are shown as spiral ribbons, the β-pleated sheets as broad, flat arrows. This enzyme is present in tears and sweat, where it catalyses the breakdown of some bacterial cell walls.
Tertiary structure
The tertiary structure of a protein is its overall three-dimensional shape and is produced as a result of the following:
- The sequence of amino acids that produces α-helices, β-sheets and bends at particular places along the chain.
- The hydrophobic nature of many amino acid side chains. Globular proteins are surrounded by water and so the hydrophobic side chains tend to point inwards.
Tertiary structure is maintained by attractive forces that arise when the amino acid chain folds and also by disulphide bridges – covalent bonds that form when two sulphur-containing cysteine residues react. Disulphide bridges occur most often in structural proteins, where they contribute to strength. I cannot over-emphasise the importance of tertiary structure for protein function. Functional proteins, such as enzymes and antibodies, must have an exact shape – and sometimes the ability to change shape – to fulfil their role in the organism. Many structural proteins depend on their tertiary structure for strength. The large number of disulphide bridges in keratin, for example, makes body structures such as hair and nails very tough.
If a protein consists of only one polypeptide, the tertiary structure is the final shape of the molecule. If, however, the protein has more than one, it has a further quaternary level of structure.
Quaternary structure
Many proteins consist of more than one polypeptide chain and sometimes also have non-protein prosthetic groups, often vital to the function of the protein. The quaternary structure refers to the three-dimensional structure, or conformation, produced when all the sub-units combine to give the final, active molecule.
Fibrous and globular proteins
The final three-dimensional structure of proteins results in two main classes of protein – fibrous and globular. Fibrous proteins contain polypeptides that bind together to form long fibres or sheets. They are physically tough and are insoluble in water. Globular proteins are usually individual molecules with complex tertiary and quaternary structures. They are spherical, or globular, in shape, hence the name. Most are soluble in water and they tend to have a biochemical rather than a structural function.
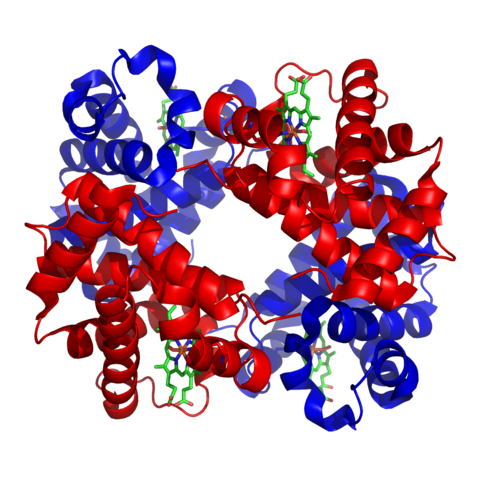
Structure of haemoglobin. Zephyris, CC BY-SA 3.0
How stable are proteins?
As the final shape of globular proteins is maintained by relatively weak molecular interactions such as hydrogen bonds, proteins are very sensitive to temperature increases and other changes in their environment. As the temperature goes up beyond 40 °C, molecular vibration increases and bonds that are holding together the tertiary or quaternary structure break, changing the shape of the molecule. This is known as denaturation. Different proteins are denatured at different temperatures. Some begin to be denatured after about 40-45 °C or even below, but many are not totally denatured until 60 °C or even higher. It is an oversimplification to say ‘organisms die at temperatures over 44 °C because their proteins become denatured’. In practice, organisms die because of a metabolic imbalance caused when enzymes work at different rates.
Proteins can also be denatured by adverse chemical conditions. Chemical that affect weak bonds alter the overall structure; even a slight change in protein shape can mean loss of function. Some proteins are particularly sensitive to changes in pH.
I will continue this series in my next post, where I will talk about how to analyse proteins and also on the chemistry of collagen.
Thanks for reading.
REFERENCES
https://www.health.ny.gov/publications/1210/
https://www.ncbi.nlm.nih.gov/pubmed/6367501
https://sciencing.com/what-is-main-purpose-of-protein-in-living-things-7317884.html
https://en.wikipedia.org/wiki/Protein_structure
https://www.nature.com/scitable/topicpage/protein-structure-14122136
https://en.wikipedia.org/wiki/Globular_protein
http://www.differencebetween.net/science/health/difference-between-globular-protein-and-fibrous-proteins/
http://www.cryst.bbk.ac.uk/PPS2/projects/day/TDayDiss/index.html
https://www.sciencedirect.com/topics/neuroscience/protein-stability
Very good post !
Posted using Partiko iOS
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.