DNA TECHNOLOGY: Cloning Genes and Making Transgenic Organisms.
The first recombinant DNA molecules were produced
in the early 1970s when bacteria were made to copy foreign DNA along with their
own, However, in 1983 an American called Cary Mulls cloned DNA without bacteria
using the relevant enzymes. This process, the polymerase chain reaction (PCR),
is gene cloning in a test tube. It allowa DNA to be amplified (copied many
times).
The idea behind PCR is very simple. You mix your original piece of DNA with the enzyme DNA polymerase in a soluton of nucleotides. Next, add some primers, short pieces of DNA which act as signals to the enzymes, effectively saying ‘start copying hee’, You then heat the DNA so that it denatures into two strands, The themostable DNA polymerase gets to work and produces two identical strands of DNA. A second cycle of reactions, the two strands become four, and so on. Typically the cycle is repeated about 20 times and within an hour you have millions of copies of the original piece of DNA.

A thermal cycler for PCR. Rror, CC BY-SA 3.0
This technique has many applicalions. Forensic scientists use it to amplify tiny DNA samples trom spots of blood or hair roots, to ottain enough material for forensic analysis by DNA profiling. PCR also allows archaeologists to study small samples of DNA hom historical material such as the preserved bodies found in peat bogs or ice graves.
DNA ligases
The sticky ends of the DNA strands produced by the action of restriction enzymes are joined together by enzymes called DNA ligases (ligate means to join/bind). The normal function of a DNA ligase enzyme is to join strands of DNA during replication. They are also involved in DNA repair. Genetic engineers use them as ‘molecular glue’ and they are essential partners for restriction enzymes. An example is T4 DNA ligase, which is made by the bacterium E. coli.
AMPLIFYING THE DNA OF A CHOSEN GENE
Armed with restriction enzymes, ligases and other enzymes, genetic engineers can isolate a single gene that they wish to clone. They can use the polymerase chain reaction (PCR) to make multiple copies of the gene so that they have enough material to work with.
USING VECTORS TO TRANSFER GENES INTO BACTERIA
Although DNA fragments can be multiplied by PCR, it is often much easier to put the DNA into organisms such as bacteria which will then adopt the new DNA as their own, and copy it for you. This is called gene cloning, or recombinant DNA technology.
All the bacteria express the gene and large amounts of the gene product can be made by culturing the bacteria in large industrial fermenters. Putting a gene from a mammal or a human into a bacterium is not an easy task. The first step is to attach the gene to a vector, or carrier. One such vector is a plasmid, a tiny circular piece of DNA that occurs in bacteria. Like restriction enzymes and ligases, plasmids can be bought commercially.
The plasmid is cut using the same endonuclease enzyme used to cut the human DNA to obtain the gene. Using the same endonuclease is important – the DNA must be cut at the same base sequence to produce complementary sticky end that will join up. The gene and the plasmid are mixed together and then a DNA ligase is added to join up the sticky ends. The DNA molecule produced is circular, like the original plasmid.
In the second step, the genetic engineer must induce the bacterium to accept the plasmid. One technique involves soaking the bacteria in ice-cold calcium chloride and then incubating them at 42 °C for 2 minutes. Nobody know exactly why this works, but it does. Bacteria which have accepted the plasmid now contain recombinant DNA, and are, by definition, transgenic organisms.
The conversion process is not very reliable: for every bacterium that takes up the recombinant plasmid, about 40 000 do not. So how do you tell which ones are recombinant? A clever trick here is to insert two genes into the plasmid: the one you want to use and one that makes the recombinant bacteria easy to detect. For instance, you can add a second gene that confers the antibiotic resistance and then culture the bacteria on agar plates containing the antibiotic. Only the bacteria with the modified plasmid will survive and grow.
An alternative is to add a second gene that codes for an enzyme that metabolises a coloured substrate. When bacteria are grown on agar plates made with the substrate, colonies that have taken up the plasmid are a different colour from colonies of non-recombinant bacteria.

Once you have identified colonies of recombinant bacteria, you can start pure cultures. Transgenic bacteria are often described as ‘sick’ because they multiply more slowly than normal bacteria: the population doubles every 30 minutes instead of every 20 minutes. Within ten hours, one transgenic bacterium can produce over a million copies of itself, each one containing a working clone of the original gene.
Modified bacteriophages also make good vectors. They reproduce by inserting their own DNA into the DNA of a host bacterium. Bacteriophages can transfer larger amounts of DNA than plasmids, but, at present, their use is limited.
TRANSFERRING GENES INTO EUKARYOTIC CELLS
Usually, a gene is transferred into a different organism so that it can be expressed, making greater volumes of product. This happens only when the gene is able to use the mRNA, ribosomes and Golgi apparatus, for protein synthesis. Some eukaryotic genes are not expressed effectively by prokaryotic cells, and so must be transferred into another eukaryotic cell. Yeast, a single-celled fungus, is often used.
Producing recombinant eukaryotic cells is more difficult than producing recombinant bacteria. The cell walls of fungal cells are a major barrier. But they can be digested away with suitable enzymes to form a protoplast, a cell without a cell wall. In this ‘naked’ state, the yeast cell will accept plasmids.
Other methods of transferring DNA into eukaryotic cells include the following techniques:
- Microprojectiles. It is possible to shoot DNA into host cells. Tiny pellets of metal are coated with DNA and fired at high speed at the target cells. Remarkably, some of the cells recover and accept the foreign DNA.
- Electroporation. This involves exposing the host cells to rapid, brief bursts of electricity to create temporary gaps in the cell surface membrane, through which foreign DNA can enter.
- Liposomes, In this more subtle method, DNA is inserted into liposomes – spheres formed from a lipid bilayer. The liposomes fuse with the surface membrane of the host cell and introduce the DNA into the cytoplasm.
- Calcium phosphate precipitation. Plasmids are mixed with calcium phosphate. As this precipitates, the grains that form contain DNA. These grains can enter cells by endocytosis.
- DNA injection. DNA can be inserted directly into a cell using a very fine pipette called a micromanipulator that avoids the inevitable hand tremor. Tracey, a transgenic sheep, was created when the gene for alpha-1-antitrypsin was manually injected into the cells of a sheep embryo. This method leaves much to chance: many embryos have to be treated before one takes up the foreign DNA.
USING RECOMBINANT PROTEINS IN MEDICINE
The protein products of cloned human or mammalian genes expressed in bacteria or eukaryotic cells like yeasts are called recombinant proteins. We saw how bovine growth hormone can be produced as a recombinant protein. Proteins important for human medicine can also be manufactured in large quantities in this way – such as human insulin. Sheep have been used to successfully produce proteines that can be used in human medicine just as is explained below.
FARMING OR PHARMING?
A new and highly experimental use of genetic engineering is ‘pharming’. This involves farming, but its objective is not to produce food. The idea is to genetically engineer crop plants and animals usually used for food so that they can produce medicines. The technique is difficult and expensive because it requires a transgenic organism that is bred to carry a human gene that makes a particular protein – it is possible, for example, to genetically engineer a cow to produce human haemoglobin in its milk. Once transgenic embryos have been produced in the laboratory, they are implanted into surrogate mothers and carried to term, resulting in transgenic cows that secrete human haemoglobin in their milk.
Milk from a cow is relatively easy to obtain in large amounts; the secreted haemoglobin is then extracted and purified. This process has been used to produce other blood components, human growth hormone and large quantities of human proteins needed for research.

Dolly, the trangenic sheep. Toni Barros, CC BY-SA 2.0
GENE THERAPY
The new technology of gene therapy promises to revolutionise medicine in the twenty-first century. The idea that it can be used to treat genetic diseases such as cystic fibrosis and Tay-Sachs disease has been around for several years, but it is taking longer than expected to develop gene therapy into an acceptable and safe treatment.
Recently, scientists have begun to look beyond the diseases caused by a defect in a single gene and are considering gene therapy as a potential treatment for all sorts of problems, from cancer and heart disease to AIDS.
THE PROCESS OF GENE THERAPY
A simple definition of gene therapy is the treatment of a genetic disease by giving individuals with the disease copies of healthy genes. Researchers use one of several approaches in gene therapy to correct the function of a disease-causing allele:
- A normal allele can be inserted somewhere into the genome. When expressed, the protein it produces is functional and makes up for the allele already there that produces a non-functional protein. This is a common method.
- A normal allele can be swapped with a faulty allele by homologous recombination.
- The faulty allele can be repaired, returning it to its normal function.
- The degree to which an allele is turned on or off can be changed.
How are genes introduced?
The most common method of gene therapy is to introduce a healthy copy of an allele into the genome to replace the faulty allele – but how is this done?
A carrier molecule called a vector must be used to deliver the therapeutic allele; it cannot get inside the host genome by itself. The most usual vector is a virus. Viruses have evolved to insert their DNA (or RNA) into genomes. If an allele for gene therapy is inserted into a viral genome and then the virus is used to infect a patient, the virus inserts the allele into the patient’s genome along with its own DNA.
The viruses used are not pathogenic – they don’t cause disease in humans – but the procedure is not without risk. An 18-year-old man in a gene therapy trial in 1999 died because of a massive allergic reaction to the adenovirus vector he was given.
Common virus vectors used in gene therapy are:
- Retroviruses. Viruses that can create double-stranded DNA copies of their RNA genomes, e.g. HIV.
- Adenoviruses. Viruses with double-stranded DNA genomes, e.g. the common cold virus.
- Adeno-associated viruses. Small, single-stranded DNA viruses that can insert their genetic material at a specific site on chromosome 19.
- Herpes simplex viruses. Double-stranded DNA viruses that infect neurons, e.g. herpes simplex virus type 1, which causes cold sores.
DNA can also be introduced without virus vectors. The simplest method is the direct introduction of therapeutic DNA into target cells. This is difficult to do and its use is very limited. Another alternative is to use liposomes, artificial lipid spheres which carry the DNA inside them. The sphere can blend with the cell membrane and take the DNA inside a cell.
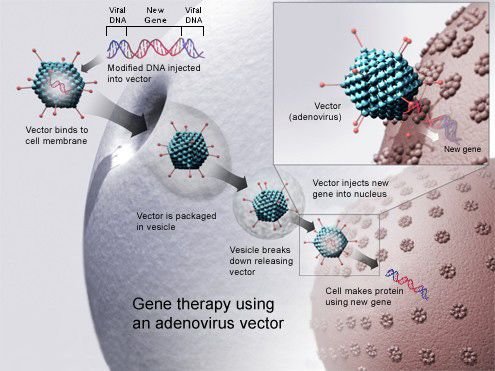
Gene therapy using an adenovirus vector. National Institutes of Health, US, Public Domain.
GENE THERAPY FOR GENETIC DISEASES
The first person to be given gene therapy was Ashanti DeSilva. She had severe combined immunodeficiency (SCID) and was treated in September 1990, when she was 4 years old. A team in the USA led by French Anderson gave Ashanti four infusions of cells containing the working gene that she lacked. Over the four months of the treatment, her condition improved.
With the help of follow-up treatments, she has now been transformed from a small girl who was constantly ill and could not leave the house, to a normal, healthy and lively teenager. She continues to do well and, apart from needing regular injections of one of the enzymes she cannot make naturally, she lives a normal life.
Early in 2000, 10 years after the first successful gene therapy trial to treat SCID, researchers in Paris managed to treat two babies, aged 8 and 11 months, with gene therapy. The babies both had SCID, but a slightly different form from Ashanti DeSilva. Their treatment involved taking out some bone marrow and selecting out a set of blood stem cells. Stem cells are the cells that differentiate into all sorts of blood cell types, including the white cells that make up the immune system. By introducing the gene that the babies were missing into these stem cells, the researchers managed to get the correct gene into all of their immune cells. Both babies developed a fully functional immune system within a few weeks and can now live normal lives. A similar process was used in the summer of 2001 on 18-month-old Rhys Evans at Great Ormond Street Hospital in London.
Thanks for coming.
REFERENCES
https://en.wikipedia.org/wiki/DNA_ligase
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3521702/
https://www.ncbi.nlm.nih.gov/books/NBK26837/
https://www.ncbi.nlm.nih.gov/books/NBK216398/
https://www.ncbi.nlm.nih.gov/books/NBK21498/
https://www.sciencelearn.org.nz/resources/527-how-to-add-foreign-dna-to-bacteria
https://www.sciencedirect.com/science/article/pii/S1389035201000666
https://www.ncbi.nlm.nih.gov/books/NBK22002/
https://mbio.asm.org/content/7/4/e00863-16
https://link.springer.com/chapter/10.1007/978-1-4615-1353-7_7
https://en.wikipedia.org/wiki/List_of_recombinant_proteins
https://www.ncbi.nlm.nih.gov/pubmed/8527840
https://www.cusabio.com/c-20272.html
https://www.britannica.com/science/pharming
https://en.wikipedia.org/wiki/Pharming_(genetics)
Your posts should be required reading before anyone watches science news, because the simple explanations of complex things would enable them to understand the meaning of the stories.
I believe you meant 1990.
Thanks!
Thanks for your nice comment, @valued-customer. You are a such a very valued customer indeed.lol
Secondly, as regards Ashanti's gene therapy. I actually made a mistake which I have just effected now; many thanks to you. I meant to write 1990 instead of 1900.
Wow. Thanks for this. This has reaffirmed a lot of what I read sometime ago. I didn't know of the mocroprojectile, electroportation, etc. Even the pharming of human haemoglobin from cow milk. Mad ooo. 🤣
Keep the information coming bro, it really is valuable.
Thanks for coming by, @benditofrancis. I'm glad you are able to learn some things from the post. Yes, I'll try to keep it up, many thanks.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
I didn't know much about this content and I liked the way you explain it, it's amazing, I didn't know that there were so many techniques even though one is not an expert in the area, it was very motivating to read it @loveforlove thanks!