The Discovery of Memory Molecules, the Petrochemical Industry and the Production of Alkene.
Chemists are beginning to discover molecules
that can be made to act like the memory of a computer, which stores binary
information by switching between two states – ‘on’ or ‘off’. The molecular
shape of certain molecules that contain double bonds alters when they undergo a
reversible photo-chemical change, and this is the ‘switch’ that chemists are
exploiting. During the photo-chemical change, the molecule keeps its structure
but changes its 3D shape. The two forms are called E-Z isomers.
When the molecule changes from an E-isomer into a Z-isomer, the distinct change in shape offers a way of storing information. For example, the E form could be designated ‘off’ (corresponding to binary digit 0) and the Z form designated ‘on’ (corresponding to binary digit 1). Given that the change easily reverses, information storage, retrieval and erasure become feasible. The hope is that, by depositing such memory molecules on an electrode, a hundred million bits of information could be stored on an area the size of a fingernail.

Methionine (Yang) Cycle. Crenim, CC BY-SA 4.0
Some of the researches carried out last year from the University of Jyvaskyla in Finland produced a molecular memory which has the capacity to remember the course of a magnetic field for a while even after which it has been switched off at very low temperatures. Such a discovery would help in improving the storage power of hard drives without expanding their original size.source
THE PETROCHEMICAL INDUSTRY
The petrochemical industry produces very many organic substances from crude oil and natural gas. About 90 per cent of the world’s output of basic organic chemicals is now derived from these two fossil fuels. Petrochemicals include substances as diverse as solvents, detergents, pesticides, insecticides, polymers, plastics, drugs, paints and textiles. Without doubt, many improvements in the quality of life since the early 1900s are a direct consequence of the development of the petrochemical industry.
Dmitri Mendeleev, who originated the Periodic Table, wrote that crude oil was too valuable to be burnt as a fuel but should be used as a source for organic chemicals. That was in 1872, and many people would argue that it is still the case.
CRACKING AND THE PRODUCTION OF ALKENES
Crude oil is a complex mixture of organic compounds. Even after the fractional distillation of crude oil, the fractions obtained are still complex mixtures (although the components of a fraction do have similar boiling points). Most of the hydrocarbons present in crude-oil fractions are alkanes, which normally are unreactive. However, alkanes can undergo the process called cracking to produce a variety of organic molecules that can be used to synthesize other, more generally useful, organic compounds.
Cracking converts saturated hydrocarbons into unsaturated hydrocarbons. In particular, a group of versatile and important unsaturated hydrocarbons, called alkenes, are made and are much more reactive than alkanes.
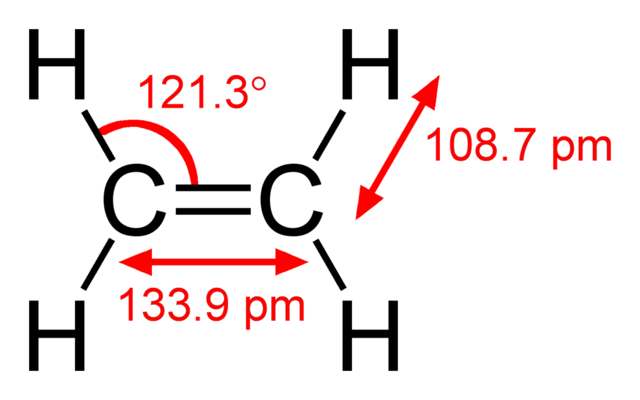
Structural formula of the ethylene (ethene) molecule, C2H4. Ben Mills, Public Domain
IMPORTANCE OF ALKENES
Why are alkenes so important to the petrochemical industry? The main reason is that the carbon-carbon double bond of an alkene molecule makes it much more reactive than an alkane molecule. So, compounds with different carbon chains or with different functional groups can readily be synthesized from alkenes.
The chemistry of the allkene functional group will be described in this post, which illustrates the wide range of substances that can be made from quite simple alkenes. Another reason for their importance is that alkenes are readily available in large amounts from the cracking of crude oil.
Alkenes and alkynes as fuels
Surplus alkenes can be used both as fuels and as starting materials for petrochemical processes. Being hydrocarbons, alkenes can burn, and so transfer energy to their surroundings.
Similarly, the alkyne ethyne (C2H2), which has a triple bond, is mixed with oxygen and burnt to produce an extremely high-temperature flame, which is used to cut iron and steel. The equipment is commonly known as the oxyacetylene torch (acetylene is the older name for ethyne).
Natural gas as a source of petrochemicals
Natural gas is an abundant resource. Even if no new gas fields are discovered, present known sources should last until about 2050. So, could natural gas be used as a source of petrochemicals as well as a fuel?
Unfortunately, natural gas is mainly methane, which is a molecule that does not contain any carbon-carbon double bonds. However, much research is in progress to find ways of using methane to make alkenes to provide an alternative supply of raw materials for the petrochemical industry. For example, in a process known as an oxidative coupling, methane is reacted with oxygen at high temperatures in the presence of an oxide catalyst. In one such reaction, methane is converted into ethene – a precursor of a large number of petrochemicals.
The coupling reaction is believed to have a free-radical mechanism. As a result, the selectivity is low, so many other products are tormed. Oxidative coupling has to be closely controlled since, with the wrong proportions of methane and oxygen, an explosive combustion reaction could occur.
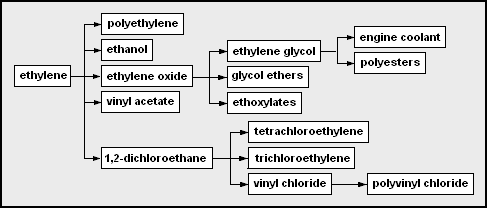
Chemicals produced from ethylene. Mbeychok, CC BY-SA 3.0
ALKENES AND ISOMERISM
Alkenes are a homologous series of unsaturated hydrocarbons that have a molecule with one carbon-carbon double bond. This means that their carbon chain has two fewer hydrogen atoms than the same carbon chain in alkanes, and has the general formula CnH2n. As already explained, it is the presence of the double bond that gives alkenes much greater reactivity than alkanes.
NAMING ALKENES
The presence of the carbon-carbon double bond (C=C) is identified by the suffix ene. So, for example, ethene has a two-carbon chain with one C=C bond, and propene has a three-carbon chain with one C=C bond. Notice that the displayed formula of ethene has been drawn with angles of about 120° between the bonds, which is their actual value. It is not essential to draw these bond angles accurately, but it is good practice, and will help you to remember them.
The name of an alkene must also specify the position of the C=C bond and the number of carbon atoms in the longest chain that contains the C=C bond. The eth, prop, but, and so on, are used to indicate the length of the carbon chain. I therefore proceed as follows:
- First, locate the longest chain that contains the double bond.
- Then, number the carbon atoms in the chain from the end that gives the lowest possible position number to the C=C bond.
The figure below shows the displayed formula of an hexene. The longest carbon chain that includes the C=C bond is shaded. It contains six carbon atoms, which means that hex is used to indicate the chain length.
The carbon atoms must be numbered so that the C=C double bond has the lowest number possible. We say it is between carbon numbers 2 and 3 (not 4 and 5), and so the name of the compound will end with hex-2-ene. Notice that only the number of the first carbon atom of the double bond is included in this part of the name. Finally, the position of the methyl side chain must be specified in the name. In this example, the methyl group is attached to carbon 4. So the full name must be 4-methylhex-2-ene.
STEREOISOMERISM
The C=C bond is stronger than the C-C bond, but it is locked in position and cannot rotate in the way that the C-C bond does. This gives rise to stereoisomerism in compounds with double bonds. Compounds that are stereoisomers of one another have the same make-up of atoms, and their atoms are bonded in the same order (same structural formula), but the arrangements of the atoms in space are different.
The figure below shows two compounds that have the same name, but-2-ene. But are they really the same? Each molecule has its own molecular shape, defined by the bond length and the bond angles within the molecule. Simple geometry shows that the length from carbon 1 to carbon 4 is not the same in both compounds. So they cannot be identical they are isomers.

The different types of isomers. Stereochemistry focuses on stereoisomers. Vladsinger, CC BY-SA 3.0
These two isomers have different physical properties. For example, the melting point of one form, cis-but-2-ene, is -139 °C, but that of the other form, trans-but-2-ene, is -106 °C. However, the chemical properties are very similar as both molecules contain the same type and number of bonds.
This type of stereoisomerisation is known as cis-trans or E-Z isomerisation. The prefix cis (Latin) means ‘on the same side’, and so the cis isomer has the two methyl groups on the same side of the double bond. The prefix trans (also Latin) means ‘on the other side’, and so the trans isomer has the methyl groups on opposite sides of the double bond. The trans isomer has the greater distance between carbon 1 and carbon 4
Nature of the double bond
Cis-trans isomers exist because the C=C bond is not free to rotate. This is unlike the behaviour of the C-C carbon bond, which has free rotation about the bond, and therefore the positions of the two methyl groups in butane are not fixed. In but-2-ene, the carbon-carbon double bond hinders rotation, and so the two methyl groups are locked in their positions.
The inability of the double bond to rotate is explained by the nature of the bond itself. Any double bond is composed of two different types of bond, the σ bond and the π bond.
The σ bond is formed by the overlap of two sp2 hybrid atomic orbitals, one on each carbon atom. The bond lies mainly in the region directly between the two nuclei. The π bond is formed by the side-to-side overlap of two 2p atomic orbitals, one on each carbon atom. As a result, the π bond lies above and below the σ bond. The two carbon atoms are thus bonded together in two different ways, with the π bond preventing rotation about the carbon-carbon bond. Note that the overlap of the atomic orbitals is less for the π bond, so the energy needed to break the π bond is less than that needed to break the σ bond.
E-Z isomerisation
Cis isomers have groups on the same side of the double bond and trans isomers on opposite sides. Look at the figure below; in this case it is not possible to tell which isomer is cis and which one is trans.
Chemists working on behalf of IUPAC have introduced a system to classify geometric isomers where it is impossible to tell whether they are cis or trans. It is called the E-Z system. The Z isomer often, but not necessarily, corresponds to the cis isomer and the E to the trans isomer.
Z comes from the German word zusammen, meaning ‘together’, and E, from the German word entgegen, meaning ‘opposite’. Looking at the geometric isomers of but-2-ene. Cis-but-2-ene is more correctly called Z-but-2-ene and trans-but-2-ene is more correctly called E-but-2-ene.
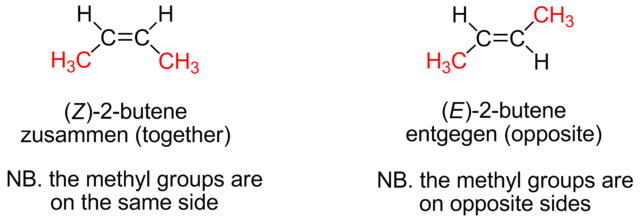
The difference between E and Z isomers. Emmmmmms at English Wikipedia, Public Domain
The general strategy of the E-Z system is to analyze the two groups at each end of the double bond. Each different group has been given a priority by IUPAC. The rules for assigning E or Z are as follows:
- Rank the two groups attached to the carbon atom at one end of the double bond.
- Rank the two groups attached to the carbon atom at the other end of the double bond.
- Look at the two higher-priority groups.
- If the two higher-priority groups are on the same side of the double bond, it is the Z isomer.
- If the two higher-priority groups are on the opposite sides of the double bond, then it is the E isomer.
Interchange of cis and trans isomers and the photochemistry of vision
The only way to change a cis isomer into a trans isomer is first to break the π bond, and thereby allow free rotation about the σ bond, and then to re-form the π bond. This can be done by a photochemical reaction using ultraviolet light.
When ultraviolet light of the correct frequency is absorbed by the molecule, the π bond is broken, to form a diradical. A diradical is a particle that contains two atoms each with an unpaired electron in its outer shell. During the time that the diradical exists, there is free rotation about the σ bond. As free radicals are highly reactive, the diradical very quickly re-forms the double bond to pair up the electrons. The result is a mixture of the cis and the trans isomer.
The property of cis-trans isomerism is not limited to compounds with carbon-carbon double bonds. Compounds with carbon-nitrogen and nitrogen-nitrogen double bonds also form geometric isomers.
PHOTOCHEMISTRY OF VISION
Light is detected on the inner layer of the eye known as the retina. The retina consists of two types of cell that can respond to light: rods and cones. Rods respond to differences in intensity of light, but not to colour. Cones respond to colour. However, both types of cell function in a similar way.
Light is detected because retinal, a molecule inside the cells of the retina, undergoes a photochemical reaction. When a photon of light reaches a cis-retinal molecule, it is changed into the trans-retinal form, and so its shape changes.
Retinal interacts with a protein responsible for producing the nerve impulses sent along the optic nerve to the brain; when the form changes from cis to trans, a nerve impulse is generated. It is not yet fully understood how the change of shape within the protein molecule produces a nerve impulse, but the energy from the light is in some way transferred, and an electrical signal results.
Thanks for reading.
REFERENCES
https://www.sciencemag.org/news/1999/03/memory-molecules
https://en.wikipedia.org/wiki/Molecular_memory
http://www.essentialchemicalindustry.org/processes/cracking-isomerisation-and-reforming.html
https://en.wikipedia.org/wiki/Alkene
https://www.britannica.com/science/petrochemical
https://en.wikipedia.org/wiki/Petrochemical
https://www.sciencedirect.com/topics/engineering/petrochemicals
https://courses.lumenlearning.com/introchem/chapter/naming-alkenes-and-alkynes/
https://en.wikipedia.org/wiki/Stereoisomerism
https://en.wikibooks.org/wiki/Organic_Chemistry/Alkanes/Stereoisomers
https://www.creative-chemistry.org.uk/molecules/isomers/e-z
https://en.wikipedia.org/wiki/Cis%E2%80%93trans_isomerism
https://socratic.org/questions/what-is-e-z-isomerism
https://www.chemguide.co.uk/basicorg/isomerism/geometric.html
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
Please join us on discord.
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Thanks.
A very interesting and comprehensive post. I really like the concept of molecular memory. Biology has used this kind of memory since it started. The classical examples would be retinal in our eyes. The light changes the bonds from 11-cis retinal to all trans retinal. Though is temporary. But other examples include phosphorylation of proteins and methylation of genes. The methylation and demethylation of genes is form of a genetic memory (epigenetics) which switches the genes on and off or may even act as subtle regulator of gene expression. I think biomemtics can also serve a way to make molecular memories. This got me thinking of very old article on using bacterial rhodopsin for making electronic or photonic devices, and even holographic memory chips. See this for example. Anyhow, really liked this post. Hope to see this field develop well in near future.
Thanks, @scienceblocks. I love your comment and I've learnt a great deal from it and also from the link you shared. Thanks, once again.