THE CHEMICAL REACTIONS OF HALOGENOALKANES, CFCS; AND THE NATURE OF OZONE.
Monohalogenoalkanes are best prepared by replacing the hydroxyl group in an alcohol with a halogen atom. A variety of reagents can be used for this conversion and some of these are concentrated hydrochloric acid, phosphorus(V) chloride, concentrated hydrobromic acid, phosphorus(III) bromide, red phosphorus and iodine.
Halogenoalkanes are saturated compounds, which means that they have no double or triple carbon-carbon bonds in their molecules. This limits the reactions available, since saturated compounds cannot undergo addition reactions. Halogenoalkanes undergo two major types of reaction, substitution and elimination. During substitution, the halogen atom is swapped for one atom or a group of atoms. The elimination reaction involves the loss of a molecule of hydrogen halide to produce an alkene.

A homemade ozone generator. Ozone is produced in the corona discharge. Science here, CC BY-SA 4.0
SUBSTITUTION REACTIONS
Polar covalent bond: The carbon-halogen bond is a polar covalent bond. The carbon atom is electron deficient, because the pair of electrons within the covalent is drawn closer to the highly electronegative halogen atom. A polar covalent bond, such as a carbon-chlorine bond, is open to attack by a species (an ion or a molecule) that seeks out electron-deficient centres.
Nucleophiles
A nucleophile is a chemical species (ion or molecule) that donates an electron pair in order to make a covalent bond with an electron-deficient centre. A nucleophile has a complete outer shell of electrons and at least one lone pair. It is this lone pair of electrons that is donated to form the covalent bond.
When we describe a species as a good nucleophile, the term ‘good’ refers to the ability of the species to donate an electron pair to form a covalent bond. The halide ions, for example, do not very easily donate the lone pair of electrons in their outer shells, and are therefore classed as poor nucleophiles.
Nucleophilic substitution
The electron-deficient, δ+, carbon atom of the polar carbon-halogen bond is attacked by a nucleophile, which donates a pair of electrons to make a covalent bond. Since a carbon atom is normally surrounded by no more than four covalent bonds (four bonding pairs of electrons), this donation must result in the breaking of another bond. When a nucleophile reacts with a halogenoalkane, a substitution reaction takes place. This reaction is called nucleophilic substitution, since the species that starts the substitution is a nucleophile.
During the reaction, a carbon-nucleophile bond is made and a carbon-halogen bond is broken. The halogen is sometimes called the leaving group, because it is the species that is lost. In this case, the leaving group must be able to gain the pair of bonding electrons. This is not a problem for a halogen atom, since it forms a halide ion, which easily supports a negative charge.
During nucleophilic substitution, heterolytic fission of a covalent bond occurs. In heterolytic fission, one of the two atoms joined by the covalent bond that breaks receives both electrons from the bond, so one atom then carries a positive charge and the other a negative charge.
Energy considerations during nucleophilic substitution
It is possible to estimate the enthalpy change of reaction by considering the bond energies of the carbon-nucleophile bond and carbon-halogen bond. Although this ignores the energy changes due to the formation of the nucleophile and the enthalpy changes due to interactions with the solvent, it is nevertheless a good way to make some predictions. The bond breaking is an endothermic process which requires the transfer of energy from the surroundings, and formation as an exothermic process, which involves energy transfer to the surroundings.
An estimate for the enthalpy change (ΔH) for the nucleophilic substitution can be calculated using the equation:
ΔH = (bond energy of carbon-halogen)- (bond energy of carbon-nucleophile)
A useful guide to the feasibility of a reaction can be obtained by looking at the enthalpy change of reaction. An exothermic reaction is often more feasible than an endothermic reaction that has a positive ΔH. In the same way, a reaction that is highly exothermic is often more feasible than one that is less exothermic. (This discussion ignores entropy considerations, which are difficult to predict in the example I’m looking at.)
This analysis of the energy changes during reactions leads to some simple ideas. Consider the reaction of hydroxide ion with fluoroalkanes, chloroalkanes, bromoalkanes and iodoalkanes. The same bond is made each time, namely the carbon-oxvgen bond. But the bond that is broken, the carbon-halogen bond, becomes weaker and weaker, and so the overall enthalpy change is more negative. So, nucleophilic substitution is easier with iodoalkanes than with fluoroalkanes. The strength of the bond to be broken in the reaction, the carbon-halogen bond, plays an important part in determining whether nucleophilic substitution will take place.
This also means the nucleophile must make a strong bond with the carbon atom rather than a weak bond, which releases little energy into the surroundings. Since the carbon-halogen bonds are quite weak, the halide ions do not act as nucleophiles in this reaction.
Mechanism of nucleophilic substitution
So far, I have only discussed which bonds have been broken and which ones have been formed. The next question to ask is: what is the sequence of bond making or bond breaking? One mechanism assumes that the two processes take place together, so that as one bond is forming the other bond is breaking. This mechanism is called SN2, since it involves two particles colliding in the slowest step of the mechanism. This mechanism is shown below. SN2 stands for substitution, nucleophilic and second order. Second-order reactions have two particles that collide in the slowest step of the mechanism. The curly arrow shows the movement of an electron pair. Primary halogenoalkanes, RCH2X (where R is an alkyl group and X is Cl, Br and I), react in this way.
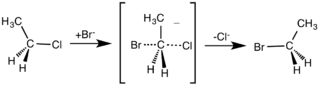
Mechanism of nucleophilic substitution. Smokefoot ,CC BY-SA 3.0
The nucleophile donates a pair of electrons to the electron-deficient carbon and at the same time the pair of electrons in the carbon-halogen bond move towards the electronegative halogen atom, eventually breaking the covalent bond and forming a halide ion. The structure of the intermediate shows that, at some time during the mechanism, both the nucleophile and the halogen atom are attached to the carbon atom. A primary halogenoalkane has one alkyl or aryl group attached to the carbon of the carbon-halogen bond, a secondary halogenoalkane has two alkyl or aryl groups, and a tertiary halogenoalkane has three alkyl or aryl groups.
SN1 nucleophilic substitution
Primary chloro-, bromo- and iodoalkanes react with nucleophiles by the mechanism known as SN2. However, tertiary chloro-, bromo- and iodoalkanes react with nucleophiles by a different mechanism known as SN1.
SN1 involves two steps rather than one and the slowest step involves only one particle – namely, the tertiary halogenoalkane. This step does not involve a collision between particles; instead the carbon-halogen bond is broken heterolytically to form a carbonium ion (often called a carbocation) and the halide ion. Once the positive carbonium ion is formed, it will react in a much faster step with the nucleophile.
It is the stability of the carbonium ion that can be formed by the halogenoalkane that determines whether the nucleophilic substitution takes place by the SN1 or the SN2 mechanism. Tertiary carbonium ions (those with a positive carbon atom surrounded by three alkyl groups) are much more stable than secondary or primary carbonium ions. Nucleophilic substitution of secondary halogenoalkanes often involves both mechanisms.
NUCLEOPHILIC SUBSTITUTION REACTIONS OF HALOGENOALKANES
Hydroxide ions, ammonia, amines and cyanide ions are all good nucleophiles and will take part in the nucleophilic substitution reaction with chloroalkanes, bromoalkanes and iodoalkanes. As already described, the carbon-iodine bond is the weakest carbon-halogen bond of the three, so iodoalkanes readily take part in nucleophilic substitution. The nucleophilic substitution reactions of chloroalkanes are much slower.
Hydrolysis
Halogenoalkanes react extremely slowly, if at all, with water. However we have already seen, they undergo a nucleophilic substitution reaction with aqueous hydroxide ions. (The hydroxide ion is a better mucleophite than a water molecule because it can more easily donate its lone pair to an electron-deficient centre.) This reaction is known as hydrolysis and involve boiling aqueous sodium hydroxide, for example, with the halogenoalkane. A hydroxyl group is substituted for the halogen atom and so an alcohol is produced. So, 1-bromobutane would be hydrolysed to form butan-1-ol:
CH3CH2CH2CH2Br + NaOH → CH3CH2CH2CH2OH + NaBr
In these reactions, the hydroxide ion is behaving as a nucleophile.
Fluoroalkanes are not hydrolysed since the carbon-fluorine bond is too strong. The case with which halogenoalkanes can be hydrolysed increases as the atomic number of the halogen increases and the carbon-halogen bond becomes weaker and longer.
Although the C-CI bond is much more polar than the C-I bond, because chlorine is more electronegative than iodine, experiments have shown iodoalkanes are hydrolysed much more rapidly than chloroalkanes. This is because the C-I bond is much weaker than the C-Cl bond.
Analysis of halogenoalkanes
Aqueous silver nitrate can be used to detect the presence of aqueous halide ions, but it will not detect the halogen in a halogenoalkane since the halogen is present in a covalent bond. To detect the halogen, the halogenoalkane must first be hydrolysed to produce aqueous halide ions.
The halogenoalkane is heated with aqueous sodium hydroxide. During hydrolysis, aqueous halide ions are produced. The hydrolysis mixture is acidified with dilute nitric acid, then aqueous silver nitrate is added. The halide ion present in the mixture is precipitated as the silver halide:
Ag+(aq) + X-(aq) → AgX(s), where X = Cl, Br or I
The colours of the precipitate are given in the table below.
Test for halide ion in the hydrolysis mixture
| Halide ion in hydrolysis mixture | Add excess nitric acid followed by aqueous silver nitrate |
| Chloride | white precipitate |
| Bromide | pale cream precipitate |
| Iodide | pale yellow precipitate |
Reaction with ammonia
Ammonia is another nucleophile, since the lone pair on nitrogen can be donated to an electron-deficient centre to make a covalent bond. Ammonia reacts with halogenoalkanes to form an amine. For example, 1-bromobutane will produce butylamine:
CH3CH2CH2CH2Br + NH3 → CH3CH2CH2CH2NH2 + HBr
There are two serious drawbacks with this reaction: ammonia reacts with one of the products (HBr), and the amine produced is also a nucleophile that can further react with the halogenoalkane. Also, this is type of reaction that gives a poor yield and many products is of little use as a synthetic method. Luckily, the reaction conditions can be manipulated to produce good yields. If excess concentrated ammonia is used, the acidic hydrogen halide product reacts with the excess ammonia to produce an ammonium salt. So, in the example, ammonium bromide would be produced:
HBr + NH3 → NH4Br
The excess ammonia also means that there is always a vast excess of ammonia nucleophile compared with the product. This ensures the maximum yield of amine. The best reaction conditions involve heating the alcoholic halogenoalkane with excess ammonia in a sealed tube.
PREPARATION OF AMPHETAMINE
The drug amphetamine is a stimulant that gives a sense of well-being and speeds up the metabolism, so that someone who takes it before doing a sport would feel less fatigued. It is prepared using a halogeno compound as an intermediate. The synthetic route employed is described below:
An alcohol, 2-phenylpropan-2-ol, is halogenated to give 2-bromo-2-phenylpropane, which then allows a nucleophilic substitution to take place using excess ammonia. The bromo compound is a synthetic intermediate that allows conversion of an alcohol into an amine while keeping the same carbon skeleton.
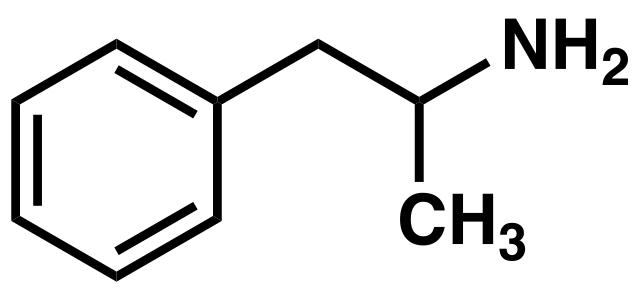
An image of the amphetamine compound. Boghog, Public Domain
Reaction with amines
Amines are nucleophiles because of the lone pair on the nitrogen atom, so they can react with halogenoalkanes to give secondary, tertiary or quaternary ammonium salts.
Reaction with the cyanide ion
Whenever possible, the starting material chosen for a synthesis already has the correct carbon skeleton. The synthesis of amphetamine illustrates this point, where the nine carbon skeleton is in place in the starting material. There are times, however, when the chosen starting material have the correct carbon skeleton and part of the synthesis is to construct the correct skeleton. An important synthetic reaction is the extension of the carbon skeleton by one carbon.
One convenient way involves the nucleophilic substitution of a halogen with a cyanide ion. In the dot and-cross diagram for the ion. The negative charge resides on the carbon atom, and it is the non-bonding pair on this carbon that is donated to an electron-deficient carbon.
A nucleophilic substitution reaction with the cyanide ion leads to the formation of a carbon-carbon bond rather than a carbon-nitrogen bond. The net effect is to increase the carbon skeleton by one carbon. The resulting functional group is called a nitrile.
The normal reaction conditions are potassium cyanide or sodium cyanide in ethanol as a solvent. An organic solvent is chosen so that the halogenoalkane dissolves and thus allows more intimate contact with the ionic sodium cyanide. 1-bromobutane reacts with potassium cyanide in ethanol to form pentanenitrile, so changing a four-carbon skeleton into a five-carbon skeleton
REACTIONS OF CHLOROFLUOROCARBONS (CFCS)
The most remarkable thing about CFCS is their complete lack of reactivity towards reagents that react with monohalogenoalkanes. CFCs do not undergo nucleophilic substitution or elimination reactions. This lack of reactivity can be explained by the inability of carbon to expand its octet and by the strength of the C-F bond.
Once CFCs are released into the environment there are no natural ways in which the compounds can be broken down. The normal decomposition processes that involve hydrolysis with water, reaction with oxygen, or bacterial or microbial decay do not take place. This naturally leads to a build-up of CFCs in the environment, and there is a risk that they will increase within the cells of living organisms. In the stratosphere, the CFCs can react, but only because of the presence of ultraviolet light.
FREE-RADICAL REACTIONS OF CFCS
CFCs contain two types of carbon-halogen bonds: the C-F and the C-Cl bonds. The C-F bond is very strong, as shown by its band energy of 439 KJ mol-1 whereas the C-Cl bond is weaker with a bond enerey of 330 kJ mol-1. This means that in the presence of ultraviolet light a C-Cl bond, rather than a C-F bond, will undergo homolytic fission to form chlorine free radical, The equation shows the homolytic fission of a CFC.
CF3Cl (g) → CF3•(g) + Cl•(g)
OZONE DEPLETION
We have seen that the chlorine free radical is very reactive and will react with many other particles. In the stratosphere, it collides with ozone molecules to form chlorine monoxide and oxygen:
Cl•(g) + O3(g) → ClO•(g) + O2
Chlorine monoxide is a free radical itself and this reaction is one of the propagation steps. The concentrations of ozone and chlorine monoxide in the stratosphere seem to confirm this reaction. It has been observed that where there is a high chlorine monoxide concentration, there is a low ozone concentration, and vice versa. Chlorine monoxide reacts with oxygen atoms present in the stratosphere to regenerate the chlorine free radical. The regeneration of the chlorine free radical completes the propagation step. The chlorine free radical can then react with more ozone. The net result of these two reactions is the conversion of an oxygen atom and an ozone molecule into two oxygen molecules:
O(g) + O3(g) → 2O2(g)
It is estimated that, on average, one chlorine free radical (hence one CFC molecule) can destroy over 100 000 ozone molecules before the chain reaction stops. It stops when the chlorine free radical collides with other molecules, such as hydrocarbons, to form hydrogen chloride that can leave the stratosphere dissolved in water. The rate of ozone depletion by the presence of chlorine free radicals now exceeds its rate of formation in parts of the stratosphere, especially over the Antarctic. The result is a ‘hole’ in the ozone layer that could have damaging consequences for humans and other life forms on Earth.
With the growing evidence of increasing ozone depletion being linked to CFC concentrations in the upper atmosphere, scientists convinced many governments to ban the use of CFC in new products, and the law has been in effect since. The hope is that without extra CFCS reaching the upper atmosphere, eventually the ozone holes over the poles will become smaller and smaller. This could take decades, because of the stability of CFCs.
THE NATURE OF OZONE
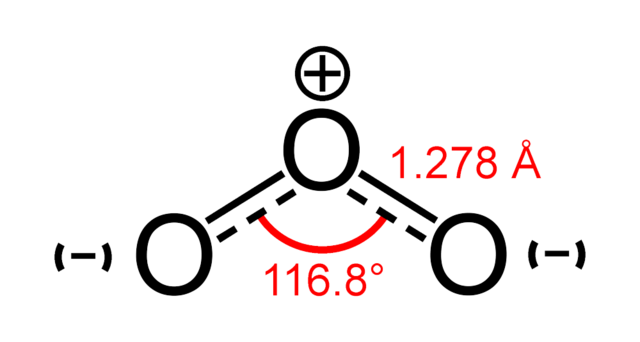
There are two forms of oxygen, O2 and O3. They are called allotropes. Ozone, O3, is thermodynamically unstable with respect to oxygen, O2:
2O3(g) → 3O2(g)
Ozone is pale blue as a gas and deep blue as a liquid. It has a sharp, irritating odour and produces headaches in humans. Even at one part per million in air, it is poisonous. But the presence of ozone in the stratosphere (a layer 12 to 50 km above the Earth's surface) acts as a protective barrier that prevents much of the high energy UV-B radiation from the Sun reaching the Earth and destroying organisms. It is a strange paradox that we are worried both about an increase in ozone concentration in the lower atmosphere and about a decrease in its concentration in the stratosphere.
Ozone is present in the lower atmosphere, formed there by the reaction between nitrogen oxides and volatile organic compounds in the presence of sunlight. It is produced along with photochemical smogs. In the past 100 years, the levels of ozone concentration have doubled over much of Europe and North America.
The effects of ozone in the lower atmosphere include an estimated 5 to 10 per cent loss in potential crop yield. It damages textiles and makes their colours fade faster. Rubber (such as car tyres) deteriorates faster, and human lungs function less well, as the airways are irritated, which causes coughing. Ozone in the stratosphere is produced by the reaction of oxygen atoms and oxygen molecules:
O(g) + O2(g) → O3(g)
Stratospheric ozone absorbs UV-B radiation by undergoing homolytic fission to form an oxygen atom and an oxygen molecule. If the ozone layer were absent or depleted, much more UV-B would reach the Earth’s surface and severely damage plant, marine and human organisms. For example, the incidence of skin cancers and cataracts would increase, crop yields would decrease and fish stocks would plummet.
CONCLUSION
After reading through this chapter, this and this, then you should know that:
Bromoalkanes, chloroalkanes and iodoalkanes can be prepared by the reaction of alcohols with concentrated hydrobromic acid, concentrated, hydrochloric acid and concentrated hydroiodic acid, generated in situ by using the sodium halides and concentrated sulfuric acid, respectively, or by the reaction of the appropriate phosphorus halides with an alcohol.
Alkanes react with chlorine, fluorine and bromine by free-radical substitution to give a mixture of halogeno and polyhalogenoalkanes. Free-radical substitution involves three steps: initiation by ultraviolet light, propagation and termination. Fluoroalkanes are very unreactive because of the strength of the C-F bond. Bromoalkanes, chloroalkanes and iodoalkanes react by nucleophilic substitution with cyanide ion to form nitriles, with ammonia to give amines and with aqueous hydroxide ion to give alcohols. Bromoalkanes, chloroalkanes and iodoalkanes can eliminate hydrogen halides to form alkenes when treated with hot ethanolic alkali.
CFCs and halogenoalkanes have weak permanent dipole-permanent dipole interactions in the liquid state, so often have low boiling points. CFCs are non-toxic and inert, and have a low boiling point. CFCs are responsible for ozone depletion in the upper atmosphere because they provide chlorine free radicals.
Thanks for reading.
REFERENCES
https://en.wikipedia.org/wiki/Haloalkane
https://en.wikipedia.org/wiki/Nucleophile
https://en.wikipedia.org/wiki/SN1_reaction
https://www.organic-chemistry.org/namedreactions/nucleophilic-substitution-sn1-sn2.shtm
https://www.chemguide.co.uk/mechanisms/nucsubmenu.html
https://study.com/academy/lesson/nucleophilic-substitution-in-haloalkanes.html
https://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html
https://pubchem.ncbi.nlm.nih.gov/compound/Amphetamine
https://en.wikipedia.org/wiki/Amphetamine
http://www.theozonehole.com/ozonedestruction.htm
https://en.wikipedia.org/wiki/Chlorofluorocarbon
https://www.chemtube3d.com/a-level-radical-reactions-cfcs-and-the-ozone-layer/
http://www.docbrown.info/page07/ASA2group7f.htm
@tipu curate
Upvoted 👌 (Mana: 20/25 - need recharge?)
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.