How To Get The Maximum Percentage Yield In The Chemical Industry, the Rate Of Reaction And the Production Of Steel.
This is sequel to my last post on Detailed Explanations on Reactions, Equations, Energy and Equilibria to a Layman. From that post, I explained some basic concepts of chemical equation, relative molecular mass, and using equations in industry. Going through the post, you should now be able to use balanced equations to calculate the masses of substances involved in reactions. We have found that 10 tonnes of Iron(III) oxide can give 7.0 tonnes of iron. The mass of iron produced is known as the yield. But chemists could never achieve this yield in a real blast furnace. Therefore we say that the calculation from the balanced equation gives the theoretical yield, which is the maximum amount if all the reactants are converted into products.There are many reasons why the theoretical yield is not achieved: reactions may not be finished in the time available, or some of the product may be lost during the purification procedure.

In industry or the laboratory, if there is more than one reactant, the reactant that is in short supply is called the limiting reactant. This is the reactant that determines the maximum amount of product that can be made. The other reactants are said to be in excess. In the case of reducing iron(III) oxide in the blast furnace, the limiting reactant will be the Iron (III) oxide because it is cheaper to have an excess of carbon monoxide.
The actual yield from a reaction can be found only by doing the reaction. From 10.0 tonnes of iron(III) oxide, it is going to be less than 7.00 tonnes of iron. Percentage yield is a convenient way of expressing how close the actual yield is to the theoretical yield.
Percentage yield = actual yield / theoretical yield × 100%
Industrial processes aim for 100 per cent yield. The closer they get, the less is the waste of raw material.
Let’s discuss about atom economy….
Even with 100 per cent yield there may still be a huge wastage of resources through the production of unwanted by-products. This is where the term atom economy comes in. It was first put forward by Barry Trost in the United States in 1991. As part of a new way of thinking called Green chemistry. He urged chemists to consider how many atoms from the starting materials actually ends up in useful products. If the atoms from the reactants do not end up in useful products, then they end up in waste products.
Atom economy = mass of desired product / total mass of reactants × 100%
Let’s consider again the reduction of the iron ore, Fe2O3.
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
The desired product is iron and in the equation 2 moles are produced:
2 × 56 g = 112 g
The masses of the reactants:
1 mol Fe2O3 (= 160 g) + 3 mol CO = 3 × 28 (=84 g)
Total masses of reactants = 244 g.
Atom economy = mass of desired product / total mass of reactants × 100% = 112 / 244 = 45.9%
So almost 54 per cent of the starting materials are lost, in this case to the atmosphere where the carbon dioxide contributes to the greenhouse effect and global warming. However, if there were an economic use for carbon dioxide then the atom economy would be 100 per cent.
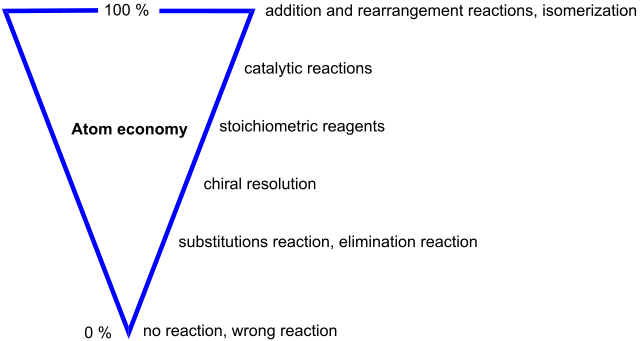
Atom economy. Astrid 91 - Own work, CC BY-SA 4.0
A brief introduction to rate of reaction
The aim of industrial chemistry is to get as close as possible to a 100 per cent yield. However, in producing any chemical economically, the yield from a reaction is just one of the factors involved. Another very important factor is the rate of reaction. It is no use having a high yield of iron in a process that is extremely slow.
As a simple definition:
The rate of a reaction is the amount of substance formed per unit of time.
The rate could be in moles per second. For industry, it is more convenient to give the rate as the mass of substance, such as kilograms or tonnes, formed per unit of time. Reactions can be very fast and uncontrolled, such as a gas explosion. Reactions can also be very slow, as in the rusting of iron.
An industrial chemist needs to control the rate of a reaction. This can either mean speeding it up or slowing it down. In industry, the rate often needs to be increased so that the amount of product formed in, say, a day is enough to make a profit.
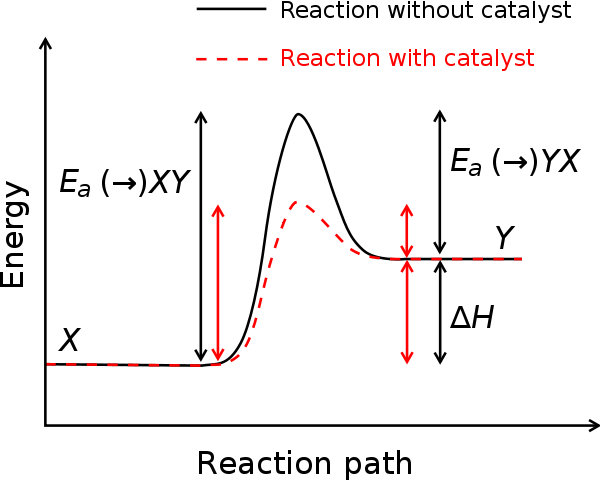
So, what are those factors that affect that rate of reaction?
Chemists control the rate of a reaction by changing the conditions of the reaction. These are some of the conditions that affect reaction rates,
- Temperature: an increase in temperature makes a reaction go faster (except for those involving enzymes)
- Pressure: increasing pressure increases the rate of reactions that involve gases.
- Concentration: increasing the concentration normally increases the rate of reaction.
- Surface area of reactant: an increase in surface area increases the rate of reaction.
- Catalyst: the addition of a catalyst usually increases the rate of reaction.
Reactions involve the rearrangement of atoms when bonds are broken and others are made, This rearrangement seldom takes place spontaneously; the particles normally need to collide with each other. All the conditions listed above will change the collision rate between particles. And if the collision rate is increased then the rate of reaction will increase, changing temperature, pressure, concentration and surface area can control the rate of reaction. However, these factors can also reduce the yield of an industrial process, so there is often a compromise between rates of reaction and yield. Remember, a major concern is the economics of the process – that is, making product quickly enough to give the maximum profit, I will look in this compromise in more detail later in this article.
Here’s another type of reaction: The chemical reactions and energy changes
All chemical reactions involve a change of energy, meaning the transfer of energy to or from chemicals in the reaction. This is crucial not only to industry, but also to the reactions of life itself. In many chemical reactions, energy is given out by the reactants as they form products, causing the temperature of the surroundings to rise. Such reactions are known as exothermic reactions.
In the blast furnace, blasts of hot air at 750 °C are blown in at the base and start a reaction between coke and oxygen:
C + O2 → CO2
This reaction is highly exothermic, raising the temperature at the base of the furnace to about 2000 °C. This is because the stored energy in carbon and oxygen is greater than the stored energy in carbon dioxide. Stored energy is known as enthalpy, symbol H. It is not possible to measure enthalpy, but enthalpy changes can easily be found by measuring temperature changes during reactions at constant pressure. Enthalpy changes are given the symbols ΔH: Δ is a Greek letter pronounced ‘delta’ and is used by chemists to mean ‘change of’.
In the coke and oxygen reaction, the energy released is 394 kilojoules (kJ) for every mole of carbon that reacts. So we write:
C(s) + O2(g) → CO2 (g) ΔH = -394 kJ mol-1
Notice that ΔH has a negative sign. This is because as the reaction proceeds, energy is lost. This energy heats up the surroundings, in this case the contents of the blast furnace. The reaction and its enthalpy changes can be shown in an energy level diagram, also called an enthalpy level diagram. State symbols should always be shown in chemical equations when dealing with energy from chemical reactions. Remember that, (s) = solid, (l) = liquid and (g) = gas.
Not all reactions are exothermic. Endothermic reactions are the opposite of exothermic reactions. In endothermic reactions energy is taken in by the reactants to form products. The energy comes from the surroundings, which lose energy and cool down.
The carbon dioxide produced at the base of the blast furnace reacts with more coke to produce carbon monoxide. This is an endothermic reaction.
CO2(g) + C(s) → 2CO(g) ΔH = +173 kJmol-1
The plus sign shows that carbon monoxide takes in energy when it is formed. As this reaction occurs in the middle of the furnace, this is one of the reasons why the blast furnace becomes cooler towards the top. This is an easy way to remember what exothermic and endothermic mean: Energy exits in exothermic reactions. Look for the minus sign (ΔH is negative). Energy enters in endothermic reactions. Look for the plus sign (ΔH is positive)
Now, what is a blast furnace and what is it used for?
The blast furnace smelts the iron ore. An ore is a naturally occurring group of minerals from which a metal, or metals, can be extracted for a profit. Smelting means that the ore is melted mixed with the other reactants and reduced to the metal. The reaction of iron ore with coke, limestone and hot air, produces the iron. The iron-making process needs high-quality iron ore that contains at least 60 per cent iron. Most iron ores contain impurities such as sand (silicon(IV) oxide, SiO2), sulphur compounds and phosphorus compounds so, if not pure enough, the ore has to be pre-refined to increase the percentage of iron.

The blast furnace lining has to last for many years to save the cost of replacing it and to minimize shutdown time for replacement work, since the loss of production may cost millions of pounds.
What are the conditions in the blast furnace?
Some of the blast furnace reactions require high temperatures, so a great input of energy is needed. Fortunately, the reaction of carbon with oxygen to give carbon dioxide provides the energy, since it is an extremely exothermic reaction. The blasts of hot air, which give the blast furnace its name, are enriched with oxygen. The concentration of oxygen, higher than in air, speeds up the reaction. The faster the rate of reaction, the more the energy produced by the exothermic reactions. In this way, the high temperatures are easier to maintain. The carbon dioxide formed near the base travels upwards through the melt and solids, and is converted into carbon monoxide. It is the carbon monoxide rather than the solid carbon that reduces most of the iron ore, since gases react faster than solids. The use of pelletised reactants ensures that carbon monoxide comes into very close contact with the iron(III) oxide. This helps to speed up the reaction because the surface area of the ore has been increased.
Finally, there are the reactions involving limestone. Limestone (calcium carbonate) decomposes into calcium oxide (lime) and carbon dioxide in the heat of the furnace:
CaCO3(s) → CaO(s) + CO2(g)
The calcium oxide removes impurities such as sand (silicon(IV) oxide, SiO,) as liquid slag. The reaction for sand is:
CaO(s) + SiO2(s) → CaSiO3(s)
Slag and iron are both liquids at the high temperatures of the furnace. Conveniently, the less dense slag floats on top of the more dense iron, so they are tapped off separately at different levels. The production of iron is a continuous process. In continuous processes, raw materials are constantly added and the products are continually removed. A continuous process is a very efficient and cost-saving way to produce materials in large quantities. By comparison, in batch processes, the reactor vessel must be closed down and reset to make another batch. This is expensive because there is ‘dead time’ when no product is being produced. However, a range of products can be made in the same vessel, although care is exercised to ensure no contamination occurs. For small quantities, the batch process is usually more cost effective.
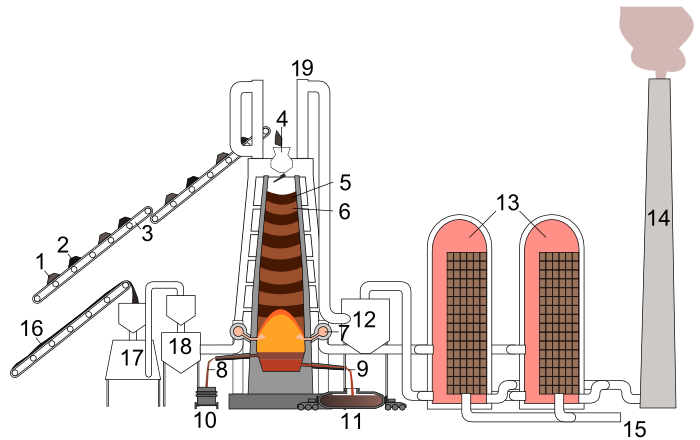
How the wastes in the smelting of Iron ore are being disposed
The waste gases and slag could cause pollution problems, but ways have been found to minimise them. Much of the slag goes to build roads and make cement. Some of it is even used to insulate houses: Rockwool, used for non-flammable loft insulation, is produced by blowing air into the molten slag to make it light and fluffy.
How steel is made from Iron.
Iron from a blast furnace is impure, brittle and not very strong, so most iron produced is changed immediately into steel. Steel is the name given to countless alloys that contain iron, carbon and, usually, small amounts of other elements. An alloy is a mixture of two or more elements, at least one being a metal. The elements are mixed when they are molten and allowed to cool down to form a uniform solid.
Alloys have different properties from the elements that form them. It is the addition of different elements to iron that changes the properties of the alloy and so makes steels such versatile materials.
Different types of steel have properties that iron lacks, giving steel a very wide range of uses such as
(a) Fractured bones are held together by permanent steel pins
(b) The Thames Barrier is mainly steel and concrete
(c) Well over half the components of a typical car are made of steel, including the body shell, clutch plates and engine.
(d) The Fruit Drop at Blackpool is made of steel to meet high safety standards.
What are carbon steels?
As the name suggests, carbon steels are alloys of mainly iron with some carbon. The sheet steel used in car bodies contains just 0.2 per cent of carbon, an amount that makes it easy to bend and shape. As the carbon content of steel is increased, the steel becomes stronger and more rigid. Most of the steel used to construct a bridge, which needs some flexibility, contains between 0.3 and 0.6 per cent carbon. The steel used in drill bits has to be very hard and contains up to 1.5 per cent carbon. It is tempting to think that by increasing the carbon content the steel would carry on strengthening. Unfortunately, this is not the case, and at just 4 per cent carbon, steel becomes very brittle.
So, what about alloy steels?
Alloy steels are steels that contain one or more other metals. These metals include manganese, tungsten, chromium and vanadium. Stainless steels are probably the best-known alloy steels, containing at least 12 per cent chromium. The chromium increases steel’s rust resistance. A common stainless steel is called 18-8 and contains 18 per cent chromium and 8 per cent nickel, a composition you will find in some cutlery. Steels that contain tungsten are very hard-wearing. Adding molybdenum (with certain other elements) enables drill bits to retain their cutting edge, even when hot. Spacecraft use titanium steel because it can withstand the high temperatures of re entry.
THE WORLD OF STEEL MAKING
The main process for converting iron into steel is the basic oxygen process. Impure iron from a blast furnace is known as pig iron and contains About 4 per cent carbon, together with other elements such as silicon, manganese and phosphorus. To convert the iron into steel, the carbon content Is lowered and other elements are removed by reacting them with oxygen. An oxygen lance is lowered into the basic oxygen furnace and oxygen is blown into the molten iron at twice the speed of sound.
The impurity elements form oxides. These are some of the reactions:
2C(s) + O2(g) → 2CO(g)
2Mn(s) + O2(g) → 2MnO(s)
4P(s) + 5O2(g) → P4O10(s)
Si(s) +O2(g) → SiO2(s)
Being a gas, carbon monoxide bubbles out of the liquid mixture. Both SiO2 and P4O10 are acidic oxides and are removed by adding lime (calcium oxide), which is a basic oxide – hence the word ‘basic’ in the basic oxygen process.

Steel Mill. Riksantikvarieämbetet / Pål-Nils Nilsson, CC BY 2.5 se
They react to form a slag that floats on top of the molten steel. These chemical reactions with oxygen are highly exothermic and generate great heat, so the reactions inside the furnace keep the contents molten. In fact, the temperature has to be stopped from becoming too high. Scrap iron and steel are added to prevent the mixture from overheating. Because as the mixture melts it takes in energy – melting is an endothermic process. If the temperature rose unchecked, then the rate of the exothermic reactions would increase, causing yet more energy to be released and making the reaction faster still. Eventually, the process would be uncontrollable and the furnace lining would be damaged.
It is the job of industrial chemists known as metallurgists to ensure that the reaction conditions of the steel making process are optimized. They also establish and monitor the best mix of elements for a particular type of steel to match customer demand.
Steel – matching supply and demand
Steel manufacture is a business, and so the production of steels must be tied closely with long-term planning to sell it. An industrial plant the size of iron and steel works cannot run economically if it has to start and stop manufacture to match short-term demands. A blast furnace works efficiently only if it is running all the time. Also, if furnace production is stopped, the furnace has to cool down and then be cleaned out. Starting it up again is a costly and difficult process that requires a lot of energy. But if the production of steel outstrips demand, the only economic solution may be to close down the whole steel works. So to keep steel works operating, the staff who are in charge of sales constantly search for new markets.
Finally, saving on energy.
We have seen that steel making has very expensive energy requirements. About 15 per cent of the cost of making steel is spent on the energy that the process consumes, so companies are continually on the look-out for ways to make savings. Building the steel works next to the blast furnace allows molten iron to be used as soon as it is made. Since the iron does not cool much between processes, its energy is not wasted. Also, the energy from hot waste gases and slag can be transferred to other parts of the operation. The waste gases can even be burnt to release energy.
Thanks.
REFERENCES
how-to-calculate-percent-yield-definition-formula
youtube video on percentage yield and calculations.
https://www.thoughtco.com/definition-of-percent-yield-605899
https://www.rsc.org/Education/Teachers/Resources/Inspirational/resources/6.6.1.pdf
http://www.greener-industry.org.uk/pages/atom/1atom_yield.htm
https://www.bbc.co.uk/bitesize/guides/z8wkh39/revision/1
https://en.wikipedia.org/wiki/Atom_economy
https://www.thoughtco.com/definition-of-reaction-rate-605597
https://www.britannica.com/science/reaction-rate
https://en.wikipedia.org/wiki/Reaction_rate
http://www.chem4kids.com/files/react_rates.html
https://www.cdli.ca/sampleResources/chem3202/unit01_org01_ilo03/b_activity.html
Factors that affect reaction rates.
https://www.open.edu/openlearncreate/pluginfile.php/22867/mod_resource/content/1/ps548_1_06.pdf
https://courses.lumenlearning.com/introchem/chapter/energy-changes-in-chemical-reactions/
https://www.sciencedirect.com/topics/engineering/blast-furnace
https://www.sciencedirect.com/science/article/pii/S0166111608707465
https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes

This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.