ALKENES: Hydrogenation, Polymerization and the Manufacture of Poly(chloroethene) from Ethene.
Alkenes can also undergo other sorts of addition reactions, such as catalytic hydrogenation. In this case, hydrogen is added to the C=C bond to make a saturated compound. The reaction mechanism is not electrophilic addition, but often involves heterogeneous catalysis (catalyst and reactant in different phases) using a nickel catalyst and high pressure.
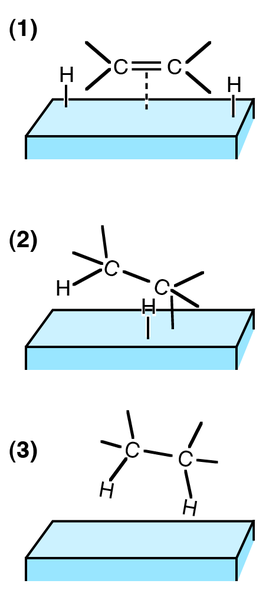
Hydrogenation is not an important reaction in the petrochemical industry because it leads to the formation of saturated (unreactive) compounds, and petrochemical feedstocks are often already saturated. As a synthetic reaction, it used when the raw material is derived from a natural source. For example, margarines are produced by the hydrogenation of polyunsaturated compounds derived from plant oils.
Working out structures
Hydrogenation is used to determine the number of C=C bonds in a compound. One mole of C=C bond requires one mole of hydrogen to fully hydrogenate it. Therefore the number of C=C bonds in the compound can be estimated by comparing the number of moles of hydrogen that have reacted with a fixed amount of compound.
UNSATURATED AND SATURATED FATTY ACIDS
Between 25 and 50 per cent of the normal energy content of the diet from a person living in the United Kingdom comes from fats and oils. This percentage is beginning to decline as people become more aware of the dangers of eating too much fatty food and are able to recognize fatty foods from improved food labeling.
There is no structural difference between a fat and an oil: they are both members of a group of organic compounds called esters. Fats are solid at room temperature and oils are liquids.
Fats are the result of the reaction between a carboxylic acid and an alcohol called propane-1,2,3-triol. The carboxylic acids are still referred to as fatty acids. Fatty acids are often known by their older, non-systematic names; these names were often derived from their sources. Stearic acid, for instance, was derived from the Greek word stear for tallow or fat.
Some of the fatty acids are unsaturated, because they have a molecule with at least one carbon-carbon double bond; some are polyunsaturated, with more than one carbon-carbon double bond per molecule; and others are saturated. Fats made from saturated fatty acids are called saturated fats and those from unsaturated fatty acids are called unsaturated fats. Medical research has recognised that unsaturated and, in particular, polyunsaturated fats are much better for your health than saturated ones. The presence of large quantities of saturated fats in a diet is believed to be part of the cause of atherosclerosis, a thickening of the arterial wall.
Vegetable oils tend to have a greater proportion of unsaturated fatty acids than animal fats and so it is believed that a diet having more vegetable oils may be better for you. There are some exceptions, however, since coconut oil contains around 90 per cent saturated fats. Margarine and low-fat spreads are made from vegetable oils. These products contain vegetable oils that have been solidified. Vegetable oils are solidified by hydrogenating some of the carbon-carbon double bonds present – essentially by making the vegetable fats more saturated. The hydrogenation reaction uses a high pressure of hydrogen in the presence of a nickel catalyst.
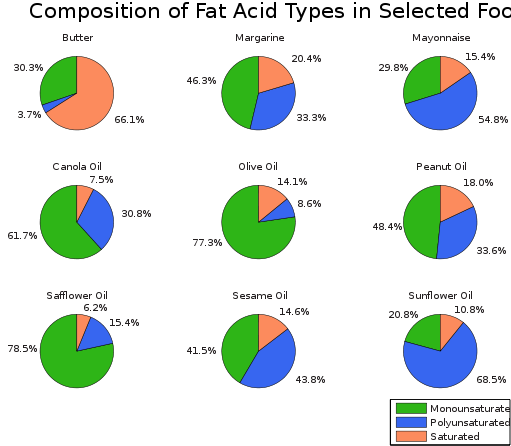
Amounts of fat types in selected foods. Xyzzy n, CC BY-SA 3.0
Natural fatty acids, such as linolenic acid, are Z or cis isomers. During hydrogenation some of the carbon-carbon double bonds react with hydrogen, but others remain intact. Unfortunately the double bonds remaining are sometimes changed from the Z form to the E form (cis to trans). These E-form fatty acids are not required by the body and there is some evidence that they may also contribute towards certain heart diseases.
It is possible that the promotion of polyunsaturated fats as healthier foods may have inadvertently introduced another health risk from E or trans fatty acids.
OXIDATIVE ADDITION
Alkenes react with cold acidified dilute potassium manganate(VII) to give a diol product (with two OH groups). The reaction involves both oxidation and addition to the double bond, so it is sometimes called an oxidative addition. During the reaction, the purple colour of the manganate(VII) ion disappears. When ethene is bubbled into cold acidified dilute potassium manganate(VII), ethane-1,2-diol (a component of antifreeze) is produced:
CH2=CH2 + H2O + [O] → CH2OHCH2OH
Notice that the oxidation is represented in the equation in its simplified form using [O]. This reaction is not used to prepare diols, since an excess of potassium manganate(VII) will oxidise the diols further. Alkaline aqueous potassium manganate(VII) will also produce a diol with alkenes. This time, a brown precipitate of MnO2 is also produced.
ADDITION POLYMERIZATION
Alkene molecules can be joined to give molecules with very long chains, known as polymers. The constituent alkene molecules of polymers are called monomers. In polymerization, thousands of monomers are combined to form a single polymer chain. An alkene forms an addition polymer when a double bond is converted into a single bond and two extra single bonds are created. The resulting polymer consists of repeating units called monomer units. For example, ethene is converted into poly(ethene), or polythene, by the action of a catalyst, high pressure and a moderate temperature.
Manufacture of poly(chloroethene) from ethene
Poly(chloroethene) is an important polymer. It is manufactured from chlorine and ethene in a series of simple chemical reactions. First, ethene is reacted with chlorine to form 1,2-dichloroethane in a typical electrophilic addition. Next, the 1,2-dichloroethane from this reaction is heated very strongly to eliminate hydrogen chloride to form the monomer chloroethene. This involves a typical reaction of halogenoalkanes.
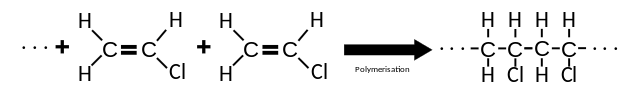
Polymerization of Vinyl Chloride to Polyvinyl Chloride. Koh Wei Teck, public domain
Hydrogen chloride is a hazardous chemical that must not be allowed to reach the atmosphere. However, it is also a source of valuable chlorine. Therefore, the hydrogen chloride generated in the second reaction is recycled to make more 1,2-dichloroethane.
Chloroethene from the second reaction is a known carcinogen, and so it too is not allowed to escape from the reaction vessel into the atmosphere. Instead, this monomer is kept in a liquid state by increasing the pressure; it is then polymerized in the presence of a free-radical initiator to form poly(chloroethene). The polymerization is highly exothermic and has a free-radical chain mechanism. To avoid explosions the rate of reaction is carefully controlled by adding ‘scavenger’ chemicals to remove any free radicals.
ALKENES AS PETROCHEMICAL STARTING MATERIALS
With their double bonds, alkenes can take part in many useful synthetic reactions. Almost all involve electrophilic additions and the introduction of functional groups to the carbon chain. It is these functional groups that allow further synthetic reactions and provide a route to a large number of organic substances.
ETHENE, PROPENE AND BUTENE
The figure below features six substances that can be synthesized directly from ethene. Most of the reactions involve electrophilic addition to the double bond and demonstrate why alkenes are of much more use than alkanes in the manufacture of petrochemicals. Alkanes have such a limited range of reactions that ethane could only be converted easily into one of the substances, chloroethane as is shown in the figure. Propene and butene are two more very useful alkenes that can be converted into a variety of useful substances.
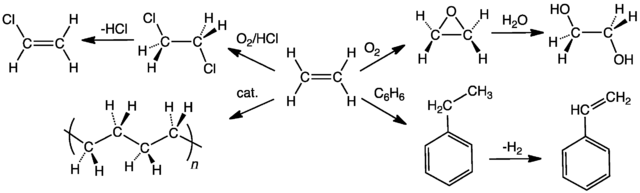
SUMMARY
After going through this post and the previous two I’ve written an Alkene (#1 and #2), you should know the following:
- Alkenes are more reactive than alkanes because of the presence of an electron-rich π bond.
- Alkenes are manufactured from long-chain alkanes by thermal or catalytic cracking.
- Alkenes are a major source of petrochemicals, such as polymers and plastics, solvents, detergents and pharmaceuticals.
- Alkenes burn in excess air to give carbon dioxide and water. Gaseous alkenes are sometimes used as fuels.
- Alkenes react by electrophilic addition with halogens to give dihalogenoalkanes, hydrogen to give alkanes, hydrogen halides to give halogenoalkanes, and water (catalyzed by acid) to give alcohols.
- The addition of an electrophile to an unsymmetric alkene forms the most stable carbocation.
- Alkenes can be oxidized by cold dilute potassium manganate(VII) to give diols.
- Alkenes are monomers that form addition polymers.
- The double bond involves the overlap of two different types of atomic orbital to form a σ bond and a π bond.
- Unsymmetric alkenes show E-Z or geometric (cis-trans) isomerism because of the absence of free rotation about the carbon-carbon double bond.
REFERENCES
https://socratic.org/questions/what-is-hydrogenation-of-alkenes
https://study.com/academy/lesson/catalytic-hydrogenation-of-alkenes-mechanism-explanation.html
https://en.wikipedia.org/wiki/Unsaturated_fat
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/saturated-fatty-acids
https://www.medicalnewstoday.com/articles/321655.php
https://en.wikipedia.org/wiki/Hydrogenation
https://wou.edu/las/physci/ch334/lecture/lect16.htm
https://en.wikipedia.org/wiki/Oxidative_addition
https://www.sciencedirect.com/topics/chemistry/oxidative-addition-reaction
https://study.com/academy/lesson/polymerization-of-alkenes.html
http://www.greener-industry.org.uk/pages/pvc/4_pvc_PMS.htm
https://www.sciencedirect.com/topics/chemistry/chloroethene
https://www.chemguide.co.uk/organicprops/alkenes/polymerisation.html
http://www.essentialchemicalindustry.org/polymers/polychloroethene.html
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
If you like what we are doing, please show your support as well by following our Steem Auto curation trail.
Please join us on discord.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.