ALKANES, THE CAR ENGINE AND ATMOSPHERIC POLLUTION #3
Carbon dioxide is not the most potent
greenhouse gas. If its concentration in the Earth’s atmosphere were to double,
the effect would probably be an increase in temperature of 1.5-4.5°C. However,
the concentration in the atmosphere of hydrocarbons such as methane,
chlorofluorocarbons (CFCs), dinitrogen oxide (N2O) and ozone (O3)
are also increasing because of human activity, and these strongly absorb IR
radiation. Clearly, the build-up of all greenhouse gases needs careful
monitoring.
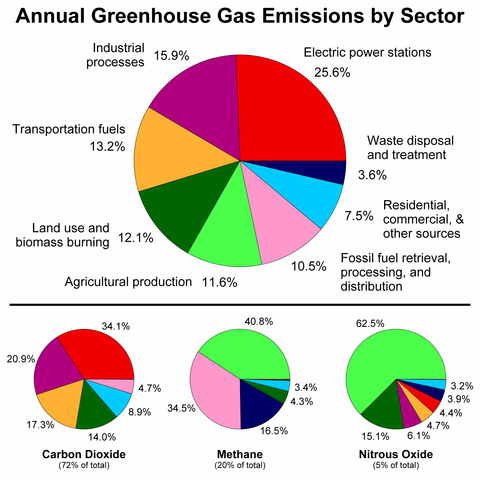
Greenhouse Gas by Sector. Robert A. Rohde, CC BY-SA 3.0
<o:p></o:p>
Methane galore<o:p></o:p>
We know from analysing the air in polar-ice
cores that the amount of atmospheric methane, a potent greenhouse gas, is
increasing. Methane-producing bacteria decompose carbohydrates, such as glucose
and cellulose, into methane and carbon dioxide, which are discharged into the
air. From glucose:<o:p></o:p>
C6H12O6
→ 3CO2(g) + 3CH4(g)<o:p></o:p>
The bacterial process is anaerobic: it does
not require oxygen, so it happens in bodies of stagnant water. For example,
paddy fields produce large amounts of methane because the water and mud that
cover the rotting vegetation provide the right conditions for anaerobic bacteria
to work. Cattle, too, produce enormous amounts of methane (each cow discharges
about 500 dm3 a day in belches from partly digested food in its
gut). And when methane reacts in the atmosphere, it produces mostly ozone,
another greenhouse gas.<o:p></o:p>
Modelling the global climate<o:p></o:p>
If we want to work out what the effects on our
climate will be of increasing concentrations of carbon dioxide in the
atmosphere, we must turn to computer models. These models use complex mathematical
equations that are based on known physical laws to make predictions about
weather and climate.<o:p></o:p>
The problem is that a model is only as good as
the information that scientists put into it. The climate is very complex, involving
complex interactions. For example, as the Earth warms up, more water vapour
will evaporate from the oceans. Water is a potent greenhouse gas, so you might
expect it to warm the Earth. However, increasing water vapour leads to
increased cloud cover, which prevents the Sun’s radiation from reaching the
Earth’s surface by day but at night traps some of the infrared radiation, keeping
air near the surface warmer. Even the height at which the clouds form has an
impact on our climate. The planet’s icecaps, too, act as giant reflectors,
reflecting the Sun’s radiation back into space; if these diminish then more
radiation will strike the Earth’s surface, producing in turn more infrared radiation.<o:p></o:p>
Another aspect that climate modellers must
consider is the effect of aerosols. These are minute atmospheric particles such
as sulfates and soot particles that are produced naturally from forest fires,
as well as by humans from fossil-fuel power stations and other industrial
activities. (Sulfate particles arise mainly from the burning of sulfur in fuels.)
These particles reflect sunlight back into space.<o:p></o:p>
At present the oceans, plants and soils absorb
half of the carbon dioxide produced by humans. Latest climate-model predictions
expect this to decrease, so as you can see very sophisticated models of how our
climate may change as a result of the greenhouse effect are required. Even a 1
°C rise in global temperature would produce unpredictable and possibly devastating
climate changes.<o:p></o:p>
This temperature is reduced to about 150 °C
when platinum is mixed with rhodium. The mixture of platinum and rhodium is
known as a three-way catalyst, because, in addition to catalysing the oxidation
of carbon monoxide, it catalyses two other reactions that involve emission pollutants.
Only 1-2 g of each element is used, but because they are coated onto a
honeycomb filter of aluminium oxide, the surface area of the catalyst is
equivalent to that of two football pitches. The use of inert supports to
increase the surface area of catalysts reduces the amount of catalyst required
and maximises the surface area available for reaction.<o:p></o:p>
UNBURNT HYDROCARBONS CxHy AND CATALYTIC
CONVERTERS<o:p></o:p>
When there is too little oxygen in a petrol
engine, unburnt hydrocarbon are present in the exhaust emissions, together with
carbon monoxide. Also, volatile hydrocarbons, such as butane, evaporate from
the petrol tank on a warm day when a vehicle is stationary. There is increasing
concern about volatile organic compounds (VOCs), especially those in the
environment. In the UK, almost 40 per cent are from fuel evaporation and
exhaust emissions. Long-term exposure to hydrocarbon emissions can impair lung
function, while even-short-term exposure can irritate the lung lining. Also,
some VOCs, such as benzene, are known carcinogens.<o:p></o:p>
The platinum in a converter catalyses the
oxidation of CxHy in the exhaust emissions. This is the
second way in which pollutants are removed in a three- way catalytic converter:<o:p></o:p>
O2, Pt
catalyst CxHy →
CO2 + H2O<o:p></o:p>
The catalyst needs to warm up before it
becomes effective, so it is during cold starts that most emissions of exhaust
hydrocarbons occur. Enough oxygen is also required through the exhaust to
oxidise CxHy and carbon monoxide. For this reason, an
oxygen sensor is fitted just before the catalytic converter to feed back information
about oxygen concentration to the vehicle’s fuel injection system.<o:p></o:p>
Evaporation of hydrocarbons can occur at any
time from the blending of petrol at the refinery to the refuelling of a vehicle
at a petrol pump. The UK has now introduced a closed system for the loading and
transport of petrol, so that all hydrocarbon vapours are trapped and recycled
before they escape. Hydrocarbon vapours still escape at the petrol pump, almost
always because of a poor seal between the tank and the nozzle.
 <o:p>
<o:p>
</o:p>
Simulation of flow inside a catalytic converter. Atif Masood (FetchCFD) , CC BY-SA 4.0<o:p>
</o:p>
OXIDES OF NITROGEN AND CATALYTIC CONVERTERS<o:p></o:p>
Air contains mostly nitrogen. Under normal
conditions, nitrogen is very unreactive. However, a petrol engine reaches
temperatures of 1000 °C, which supplies enough energy to split the very strong
triple bond in nitrogen. Nitrogen then reacts with oxygen to form nitrogen
oxides (NOx) – mainly nitrogen monoxide (NO).<o:p></o:p>
NO can be further oxidised in the air to give
nitrogen dioxide (NO2). While NO is colourless, NO2 is
brown; when the atmospheric conditions allow, this can build up as a brown haze
in large cities. NO2 contributes to acid rain, reacting with water
to form nitrous and nitric acids:<o:p></o:p>
2NO2(g) + H2O(l)
→ HNO2(aq) + HNO3(aq)<o:p></o:p>
NO2 also catalyses the oxidation of
sulfur dioxide in the atmosphere and causes respiratory diseases such as
bronchitis. The beginning of this series mentions the build-up of nitrogen
oxides in winter smogs. Nitrogen dioxide, in particular, often far exceeds
World Health Organization guidelines and is linked to more deaths occuring than
are usual.
The third way in which a three-way catalytic
converter works is to reduce NOx back to nitrogen and oxygen, this
time using a rhodium (Rh) catalyst:<o:p></o:p>
2NOx(g) → N2(g) + xO2(g)<o:p></o:p>
Too much oxygen passing from the engine into
the catalytic converter reduces the efficiency of this reaction. So, achieving the
correct fuel-air mixture is critical to the efficient function of a catalytic converter,
hence the need for an oxygen sensor in the exhaust system.<o:p></o:p>
HETEROGENEOUS CATALYSIS AND CATALYST POISONING<o:p></o:p>
The catalysts in converters are always solids,
and the reactants are always gases. So the catalysts are said to be heterogeneous
catalysts, because they are in a different physical state to the reactants. The
reactants are adsorbed onto the catalyst surface, which means they weakly bond
to it. This holds the reactant molecules close together and also allows their
covalent bonds to weaken. Adsorb is the word used when reactants are weakly
bonded to a surface. Do not confuse this with absorb, used for substances that enter
the material like a sponge soaking up water. This provides the alternative
route of lower activation energy. New bonds form and the product molecules are
desorbed.<o:p></o:p>
The strength of the weak bonds formed at the
catalyst surface with the reactant molecules and product molecules is critical
to the efficient function of the catalyst. If the bonds formed are too weak the
reactant molecules will not be held in place, and if the bonds are too strong
the product molecules will not be able to leave. This is why platinum and
rhodium are good catalysts to use in catalytic converters. Tungsten, on the
other hand, forms bonds with reactants that are too strong, while those formed
with silver are too weak.<o:p></o:p>
Just two tanks of leaded petrol are enough to
render a catalytic converter useless. This is called catalyst poisoning. Lead
poisons the catalyst because it is adsorbed more strongly than the reactant
molecules and so it blocks the active sites at which the reactants bond. (It
was for this reason that the US government made it illegal to use leaded petrol
in cars fitted with catalytic converters, and required that the opening to the
petrol tank in these cars be made too small to take a leaded-petrol nozzle.)<o:p></o:p>
SULPHUR OXIDES, ACID RAIN AND HOMOGENEOUS CATALYSIS<o:p></o:p>
Most emissions of sulphur oxides, which are
toxic, are from the burning of sulphur in fossil fuels. Sulphur dioxide is produced
in large quantities by the reaction:<o:p></o:p>
S (in fuel) + O2(g)
→ SO2(g)<o:p></o:p>
and can be further oxidised into sulfur
trioxide in the atmosphere:<o:p></o:p>
2SO2(g) + O2(g)
→ 2SO3<o:p></o:p>
Nitrogen dioxide can act as a catalyst in this
reaction, which is another reason why nitrogen dioxide emissions should be
reduced where possible. As the physical state of the nitrogen dioxide is the
same as that of the reactants, it is called a homogeneous catalyst.<o:p></o:p>
Dilute solutions of sulfuric acid in rainwater
are the main cause of acid rain. (NOx in the atmosphere also
contributes to acid rain by forming nitrous and nitric acid solutions.) Acid
rain has had serious and far-reaching consequences, killing trees in forests
and lowering the pH in lakes so that fish die. Burning petrol and diesel in
vehicle engines is not the main contributor to sulfur dioxide in the atmosphere;
it accounts for only 2 per cent of emissions in the UK. By far the worst
culprit is the burning of coal in power stations.<o:p></o:p>
Sulfur dioxide also poisons the catalyst in catalytic
converters by forming strong bonds with the active sites at the surface.
Fortunately, mixing the catalyst with aluminium oxide alleviates this because
the sulfur dioxide is held in preference by the oxide. The normal conditions of
engine running are oxidising, but during acceleration the conditions in the
converter allow the sulfur dioxide to be reduced to hydrogen sulfide, which is
then expelled and so the catalyst is not permanently poisoned.<o:p></o:p>
PHOTOCHEMICAL SMOG<o:p></o:p>
The word 'smog' was first used to describe the
combination of smoke, fog and sulfur dioxide that used to build up in London and
cause hundreds of extra deaths. The Clean Air Act of 1956 made this type of
smog a thing of the past in the UK. However, photochemical smog caused by
exhaust emissions from vehicles is a problem in many urban areas. This is also
called stummer smog; Los Angeles and Beijing are two infamous examples. The atmosphere
around a large city forms a vast mixing bowl for chemical reactions, and
sorting out just what causes photochemical smog has not been easy. Even now, it
is not fully understood.
 <o:p>
<o:p>
</o:p>
LIGHT AND THE FORMATION OF SECONDARY POLLUTANTS<o:p></o:p>
Any pollutant in an exhaust emission is called
a primary pollutant. A secondary pollutant is formed in air as a result of the
chemical reactions of a primary pollutant. Sunlight supplies the energy to
initiate the reactions that form the secondary pollutants ozone (O3)
and organic nitrates, such as PAN. Reactions in which the energy is supplied by
light are called photochemical reactions. NO2 absorbs photons of
ultraviolet light that supply the energy (hf) to split one of the
covalent bonds holding N and O together:<o:p></o:p>
NO2 (g) →
NO(g) + O(g)<o:p></o:p>
The splitting of the covalent bond is sometimes
called bond fission. There are two ways in which a covalent bond can split:
heterolytic fission homolytic fission. In heterolytic fission, both electrons
from the bond go to one atom. The atom that gains an electron becomes
negatively charged, while the atom that loses an electron becomes positively
charged.<o:p></o:p>
In homolytic fission, when the bond breaks the
bonding pair of electrons are equally shared, so that each atom in the bond
gains one electron. The atoms are not charged because the number of protons is balanced
by the number of electrons.<o:p></o:p>
Free radicals result from homolytic fission.
Free radicals are species with an unpaired electron, which often makes them
highly reactive. So, in our example, X and Y are both free radicals. The unpaired
electron on a free radical can be shown by a raised dot, X•. Taking the
homolytic fission of NO2 by light. Both NO and O are free radicals.
In fact, atomic oxygen has two unpaired electrons and is called a diradical. Oxygen
atoms are very reactive; one of their reactions involves the production of
ozone (O3):<o:p></o:p>
O(g) + O2(g)
→ O3(g)<o:p></o:p>
The presence of ozone in the stratosphere is essential to prevent too much ultraviolet light penetrating to the lower atmosphere (troposphere). However, its build-up close to the Earth's surface is dangerous as it causes respiratory problems, and in high concentrations it produces coughing and nausea. It is also involved in a series of other reactions with unburnt hydrocarbons, which produce yet more ozone and a group of very unpleasant molecules called organic nitrates.
REFERENCES
https://www.epa.gov/ghgemissions/overview-greenhouse-gases
https://en.wikipedia.org/wiki/Greenhouse_gas
https://en.wikipedia.org/wiki/Methane
https://scied.ucar.edu/methane
https://en.wikipedia.org/wiki/Climate_model
https://www.gfdl.noaa.gov/climate-modeling/
https://prezi.com/sp-kbyuw0v4v/methane-and-unburnt-hydrocarbons-consequences-and-resolutions/
https://en.wikipedia.org/wiki/Unburned_hydrocarbon
https://en.wikipedia.org/wiki/Catalytic_converter
https://www.explainthatstuff.com/catalyticconverters.html
https://www.mdpi.com/2073-4344/5/1/145/htm
https://en.wikipedia.org/wiki/Heterogeneous_catalysis
@tipu curate
Upvoted 👌 (Mana: 25/30 - need recharge?)
According to the Bible, Bro. Eliseo Soriano: Does the sending of apostles have a limit?
Watch the Video below to know the Answer...
(Sorry for sending this comment. We are not looking for our self profit, our intentions is to preach the words of God in any means possible.)
Comment what you understand of our Youtube Video to receive our full votes. We have 30,000 #SteemPower. It's our little way to Thank you, our beloved friend.
Check our Discord Chat
Join our Official Community: https://steemit.com/created/hive-182074
I note that you had problems with the format of the publication...If you have doubts I can help you, send me a DM to the discord
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.