Who Discovered Electron: History of Electron
Electrons are a subatomic particle, symbolized by e or β. Its electric charge negative is an elementary charge. Electrons belong to the first generation of the lepton particle family, and are generally considered elementary particles because they have no known component or sub-structure.
Electrons play essential roles in many physical phenomena, such as electricity, magnetism, chemistry, and thermal conductivity, and they also participate in gravity, electromagnetic, and weak interactions.
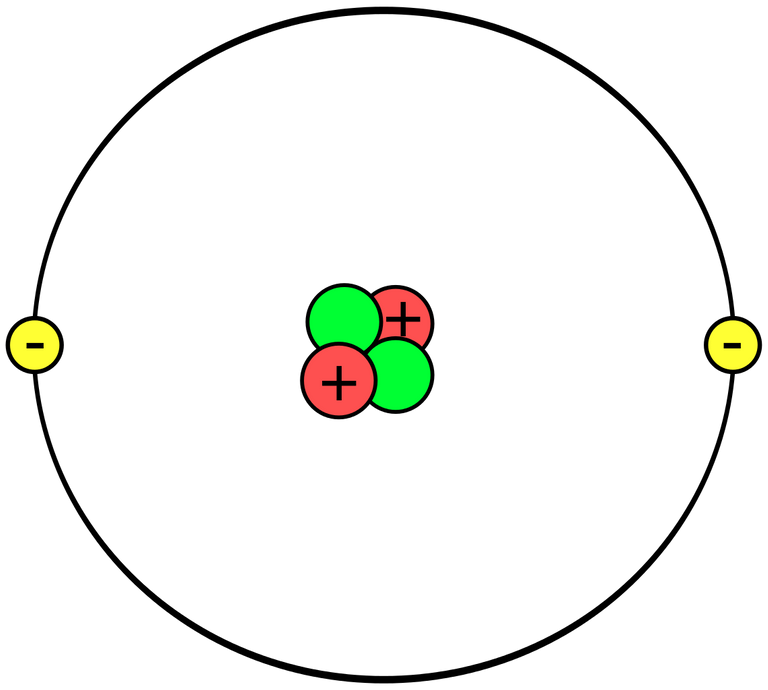
The electron is a sub-atomic particle (or fundamental particle - a small particle forming atoms and cannot be broken into smaller parts). The electrons in an atom surround the nucleus which is made up of protons and neutrons in an electron configuration (arrangement of electrons of an atom).
The word electron was coined in 1894 by Johnstone Stoney (an Irish physicist) and is derived from the Latin electrum or the Greek electron meaning amber (fossil tree resin). An electrostatic charge can be generated by rubbing hard translucent fossil resin with wool and the electrostatic charge phenomenon is normally demonstrated to elementary students.
Electrons carry a negative electrical charge and when they move, they generate an electric current.
Electrons are very small and have a mass of 9.11 × 10–31 kg, which is about 1/1836 of the mass of a proton; It has a radius of 2.8179 × 10–15 m, and has a negative electric charge of −1.6 × 10–19 coulombs.
Atoms play an important role in chemistry because the electrons of an atom are determined by the way in which atoms interact with other atoms (chemical reactions) and this process lags behind the chemical properties of various materials.
The electron as a unit of charge was established in 1874 by Johnstone Stoney, who also invented its name.
The discovery that for many, the electron was a sub-atomic particle was discovered in 1897 at the Cavendish Laboratory in Cambridge J. J. Thomson, when he was studying cathode rays. Influenced by Maxwell, and the discovery of X-rays by Röntgen, he stated that cathode rays were present and negatively charged particles.
Periodic law states that the chemical properties of an element repeat themselves periodically as seen in the periodic table of the element. Many satisfactory explanations for this were not given until 1913, when Henry Mosley introduced the concept of atomic number and explained the periodic law with the number of protons of each element in each nucleus.
In the same year, Niels Bohr showed that electrons and not protons, in fact, are behind the periodic table behavior. In 1916, Gilbert Lewis and Irving Langmuir went ahead and explained the chemical relationship of the element by electronic interactions.
In a simplified way, it is possible to say that electrons in an atom are present in several electron circles around the nucleus and they move there (when the element is stationary). Each electron shell is given numbers 1, 2, 3 and so on, starting with a nearest nucleus. Each shell can hold a maximum of electrons. The distribution of electrons in the shell is called the electron configuration.

Source
With the outermost shell electrons, the maximum possible electrons are less reactive. External shell elements with less than maximum electrons are more reactive.
The motion of the electron around the nucleus is a controversial subject. It is not possible to say that electrons exhibit motion in the physical sense of the word. Rather, the electron appears inside and outside the existence at various points around the nucleus and we cannot estimate the exact position of an electron. This is certainly a difficult idea and is further explained by the Heisenberg uncertainty principle (quantum mechanics).
The electron belongs to a class of sub-atomic particles called leptons (fundamental components of matter) which are fundamental particles. The term "particle" is difficult because quantum mechanics shows that electrons also behave like a wave - this phenomenon is called wave-particle duality.
Nobel Prize in Physics
Thomson received various honors, including the Nobel Prize in Physics in 1906 and a Knighthood in 1908. He also had the opportunity to see many of his close associates win the Nobel Prize, including Rutherford (Chemistry) and Aston (1922) in chemistry.
Sir George Thomson, son of JJ Thomson, was a noted physicist by himself and won the Nobel Prize in 1937. On the 70th anniversary of the discovery of electrons, he wrote about that discovery and subsequent developments where electrons were sometimes discovered to function.
References for further study:
@orion7


good writing bro.
Nice.
You got a 97.13% upvote from @minnowvotes courtesy of @orion7!
flagged for bid bot abuse, @steemflagrewards
Everyone is responsible for purifying the community
净化社区,人人有责
my 2% downvote just is a summons that replace punish
我2%的downvote,只是传票,不是惩罚
If you support my work, you can give me some praise
如果支持我的工作,可以给我点赞
If you find my mistake, you can give me a valid upvote (0.02sbd) on this comment. I will arrange a time to review or provide relevant evidence as soon as possible.
如果发现我判断错误,可以给我有效点赞(0.02SBD),我会安排时间,尽快复核或提供相关证据.
This post earned a total payout of 0.266$ and 0.200$ worth of author reward which was liquified using @likwid. To learn more.