Mitochondrial medicine: Fact or fiction? Chapter I –Mitochondria, our best friends!
Mitochondria are those structures within our cells that are producing the most energy. More than 90% of cellular energy is produced by them. Besides energy production, many other fundamental functions are associated with them. The spectra reach from fatty acid, porphyrin or amino acid metabolism, to the regulation of calcium homeostasis or the programmed cell death, Apoptosis.
Mitochondria in action! Thanks to @remotehorst23. No Copyright cc0. - unrestricted use allowed
Within the last couple of years, so-called mitochondria medicine has achieved a lot of popularity. It is assumed that damages in the mitochondrial structure are the origin of numerous health problems. Therefore, mitochondrial medicine offers novel diagnostic as well as therapeutic concepts.
In fact, many diseases are associated with alterations in normal mitochondrial function. These are besides psychological disorders (autism, depression, burn-out or Alzheimer's disease) also diseases, such as cancer, diabetes or fatigue [1-3].
At the moment, there is no promising concept for the treatment of mitochondrial diseases in general medicine. Therefore, mainly specialized medical practices and laboratories offer these services.
Let's shed light on the subject of mitochondrial medicine to see whether it could have merit even for "ordinary" people.
In the first chapter (today) we will discuss the following questions:
- What are mitochondria and where are you they from?
- How do they work and how do they contribute to our lives?
- And are they really that important?
In the second chapter, we will have a closer look at mitochondrial dysfunctions and mitopathies. There we will discuss the following aspects:
- What is a mitochondrial disease?
- Who suffers most?
- Are there experimental models?
- Is the role of mitochondria in diseases scientific proven?
- How could problems be noticed?
- What can you do if your mitochondria are sick?
Eventually, in the last chapter (part III), we will go further into detail and discuss technical aspects to research mitochondrial diseases.
But now let’s start with Chapter 1 and figure out what mitochondria actually are.
Mitochondria are organelles. Organelles are structures within your cells which are surrounded by membranes and therefore separated from the remaining cellular space [4]. These barriers allowing the creation of areas for specialized reactions. So-called compartments were built. The membranes around these compartments are composed as lipid bilayers. A lipid bilayer is the general membrane architecture of cells. The mitochondria (similar to chloroplasts), however, bearing two of these lipid bilayers [5]. The inner lipid bilayer of the mitochondria further has specialized lipids, such as cardiolipin [6]. This kind of architecture is highly similar to bacteria [7]! Moreover, mitochondria (as well as chloroplasts) carrying their own genome (DNA). Comparable to bacteria, this genome is circular (genomes of animals are usually linear). Additionally, the mRNAs which are transcribed from the DNA are polycistronic [4, 8]. This means that one mRNA carries the information of more than one gene and can, therefore, produce more than one protein. (Please see „The dogma of molecular biology" for more information). Animals usually don’t have polycistronic mRNAs. Moreover, the tRNAs in the mitochondria are also bacteria-like [8]. Nevertheless, the mechanism of transcription and translation, as well as the construction of the ribosomes unique in mitochondria and can neither be compared to animals nor bacteria [2, 9, 10].
Table 1: Animals, mitochondria, and bacteria in comparison (Rough selection!).

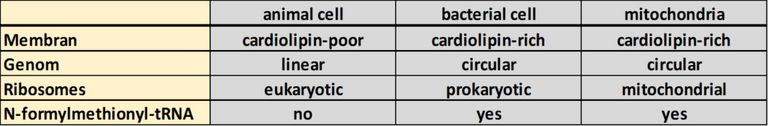
In short:
Yes, my highly regarded reader, mitochondria are with high probability, except for some differences, originated from bacteria. The hypothesis is that they entered our cells billions of years ago (endosymbiont hypothesis, Fig.1)[5].
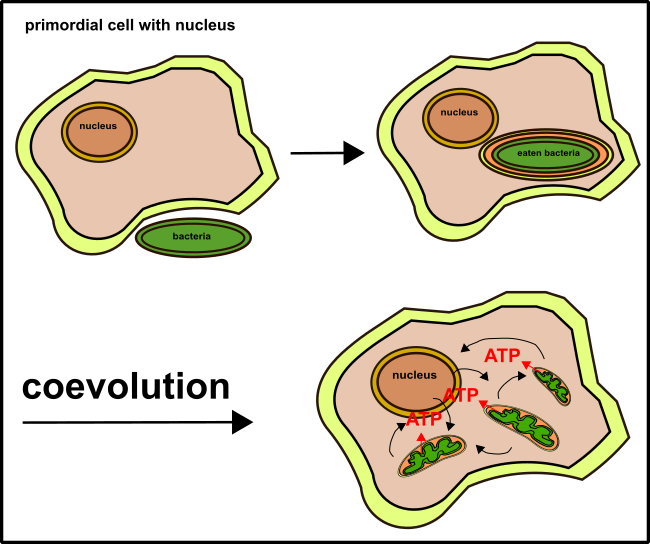
Fig. 1: The endosymbiont hypotheses. For explanation see text. Made by Chapper - unrestricted use allowed
Same holds true for chloroplasts. While both cells were fused (our ancestor cells and the bacterias), some parts of the membrane from the outside were transferred into the cell. This is the reason why mitochondria and chloroplasts bearing two lipid bilayers (Fig.1).
The entire procedure occurred at about 1.5 billion years ago [11]. Our mitochondria were used to belong to the α-proteobacteria [10]. Today bacteria such as Bradyrhizobium japonicum [5] or acetic acid bacteria are representatives of the α-proteobacteria. Bradyrhizobium japonicum, for instance, is responsible for the nitrogen fixation in soybeans. And the acetic acid bacteria are needful while making Kombucha.
But why did our ancestor cells tolerated this invasion?
Probably, because of severe energy problems. α-proteobacteria (or mitochondria) presumably have alleviated these problems with their ability for oxidative phosphorylation (OxPhos) [3].
Oxidative phosphorylation is a mechanism I often talked about before (please see here or here). It is the transfer of electrons stored in nutrients onto oxygen. As a consequence ATP is produced. The production of ATP is realized because of proton pumps which are activated by the electrons. You can compare this with a hydroelectric power station. However, in mitochondria, electrochemical forces (not electromechanical forces as in the case of hydroelectric power stations) are created which activate the enzymes for ATP generation [13]. And these forces are of high need because the negatively charged ADP and negatively charged phosphate repel each other. With the proton motive force, however, both (ADP and phosphate) are squeezed together to form the highly energetic ATP. ATP for its part is the molecule that drives (almost) all cellular events. For this, the phosphate is detached from ADP again and releases the energy that was stored in ATP before. This free energy is the force that keeps your body and mind in action [13-15]. Examples for these reactions are anabolic reactions (e.g. synthesis of proteins or fatty acids), movement (such as muscle contraction), but also DNA replication, repairing, recycling, signal transduction… In short: Everything to survive! It is the everlasting struggle to prevent the cell from achieving the thermodynamical equilibrium, which means death! Death can only be prevented by a constant intake of energy [13]. That’s why mitochondria are of such huge importance.
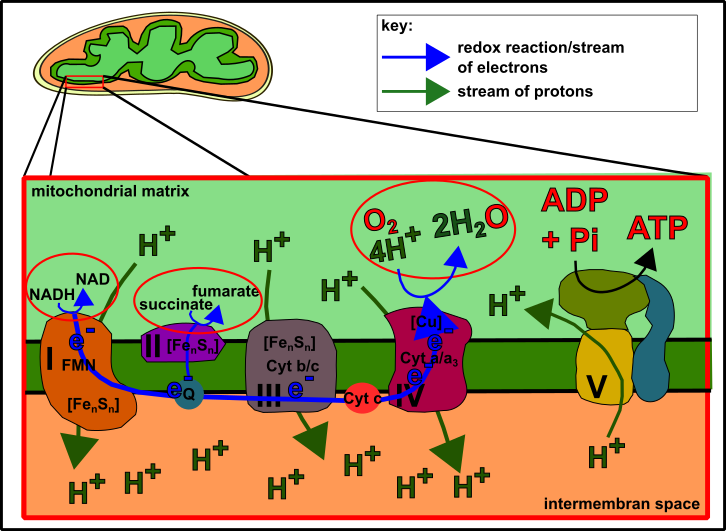
Fig.2 The mitochondrial respiratory chain (also see here). Made by Chapper - unrestricted use allowed
But how received the cell energy before the proteobacteria entered? Good question! Probably by simply doing glycolysis (Fig.3). Glycolysis is a relatively inefficient strategy for ATP generation. In principle, glycolysis can be described as a series of enzymatic reaction finally leading to little amounts of ATP. Further products are pyruvate and NADH. NADH is the usual carrier of electrons stored in foods (see here). Cells with mitochondria using the electrons within NADH for energy production. In the absence of mitochondria, the NADH accumulates and glycolysis stops. Therefore, the NADH must be recycled back to NAD for the next round of glycolysis. This is carried out by reducing pyruvate to lactate [13, 15-17].
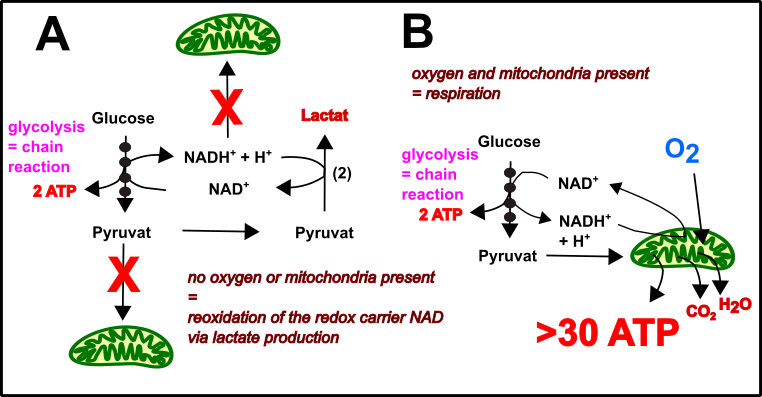
Fig.3 Glycolysis & Mitochondria: A perfect energy production unit! Made by Chapper –unrestricted use allowed
Since the mitochondria are part of "our" cells, we have the opportunity to [13, 16]:
feed electrons in our respiratory chain and receive much higher amounts of ATP.
further deplete pyruvate and thus releases much more electrons and therefore more energy.
3. decrease the acidification within and around the cell because pyruvate is no longer reduced in high amounts to lactate.
Moreover, further advantages were achieved (Fig.4)[15]:
- The cells were now able to efficiently consume fatty acid for energy production.
- Also, amino acids now served as fuels.
- Additionally, the production, conversation, and depletion of various amino acids were possible.
- Other metabolic pathways such as ketone body formation were invented and gave the body higher flexibility to various situations of nutrients supply.
- Also, the production of certain vitamins (such as B12) was no problem any longer.
- And even the detoxification of the cell was improved.
- …
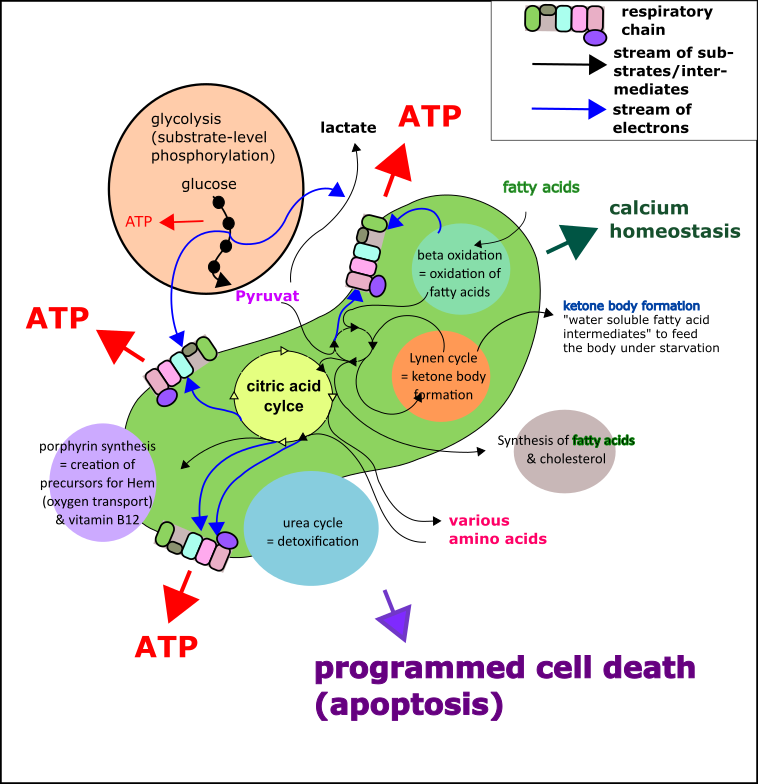
Fig.4 Small selection of mitochondrial pathways (green structure in the middle of the picture). Please note that this is only a rough overview. Details have been omitted to reduce confusion. Made by Chapper – unrestricted use allowed
The mitochondria were, therefore, innovatory benefits to our ancestors (with real nucleus = eukaryotes) they never had before.
And what were the advantages to the mitochondria [2, 15, 18]?
- The mitochondria became part of the cellular homeostasis. Protected from the outside it was the first time that they had constant conditions (constant environment).
- The mitochondria also had a constant nutrient supply (constant supply).
- Moreover, the mitochondria sourced a lot of genes into the nucleus of the host. Therefore, there were just low amounts of DNA remaining to handle (outsourcing/work sharing).
However, with these features, some problems arose. One of these problems was the incomplete transfer of electrons on oxygen. If this happens oxygen turns into highly reactive species that harm the cellular architecture.
Because of the fact that these reactive oxygen species (ROS) (Fig.5) leading to many problems, the cells were forced [19]:
to optimize the transfer of electrons on oxygen
to remove all the ROS as fast as possible.
The first case (efficient electron transport) is realized by the structure and integrity of the respiratory chain. Please have a look at Fig.1 and the description here again. All the proteins that pump the protons through the membrane are constructed and interconnected in the most efficient way. Plenty of factors such as metal ions (embedded in iron-sulfur-clusters), electron carriers (such as ubiquinone or CoQ10) floating in the membrane and proteins like the highly conserved cytochrome c doing the best they can to optimize the proton flux, which means a lot of ATP. Further, they organize an almost perfect transmission of electrons to oxygen on complex IV [1, 2, 3, 13, 16].
Unfortunately, this scenario is hard to achieve because many other factors lowering the efficiency of the respiratory chain, e.g.
- Varying nutrient supply: On one day you have more and on another day fewer nutrients. Because of this the amount of electrons entering the respiratory chain also varies. The cell, however, produces a certain amount of respiratory chain proteins. Sudden changes are hard to compensate. Increased ROS formation is possible [16].
- Bottlenecks in the flow of electrons: If too many electrons enter the respiratory chain, they can be "stauched" on their way to complex IV. As a result, electrons are "discharged" on oxygen (incompletely) via complex I & III, forming ROS [20].
- Mutations in the respiratory chain or in factors involved in the construction of the chain: Efficiency is everything with electron transport! If one of the factors is not 100% accurate problems might occur, which means ROS formation [1, 2].
- Lack of supply with respiratory chain components: If there are problems in the supply with minerals, vitamins or even proteins and peptides, then overload and incorrect transmission are hard to avoid. This, in turn, leads to ROS again [1-3, 16].
Exactly for this purpose, the cell has invented counteracting strategies [19]:
- SOD: SOD stands for superoxide dismutase. SOD is an enzyme that takes care of the removal of one of the most abundant and short-living oxygen radicals, the so-called superoxide radical. After SOD reacts with the superoxide radical hydrogen peroxide is formed (Fig.5A).
- Catalase: Even though hydrogen peroxide is less reactive than the superoxide radical it is still highly reactive and quite stable. Therefore, hydrogen peroxide must be removed as well and this is the job of catalase (Fig.5A).
Primary ROS target within proteins: Sulfur! In the cytosol sulfur groups of proteins are reduced [21, 22]. But hydrogen peroxide, for instance, is able to oxidize these sulfur groups. The results are disulfide bonds within the proteins (Fig.5B). As a consequence, the proteins are changing there structure and function. They are no longer active [21, 23]. One of the worst cases is the formation of aggregates. Hallmark diseases such as Alzheimer's disease or Parkinson are protein aggregates [24, 25]. Thus protein aggregates are severe threats for cellular health. Nonetheless, for cases like this, the cell has the glutathione system (GSH/GSSG), which removes these problems (Fig.5B)[13, 19, 21, 23].
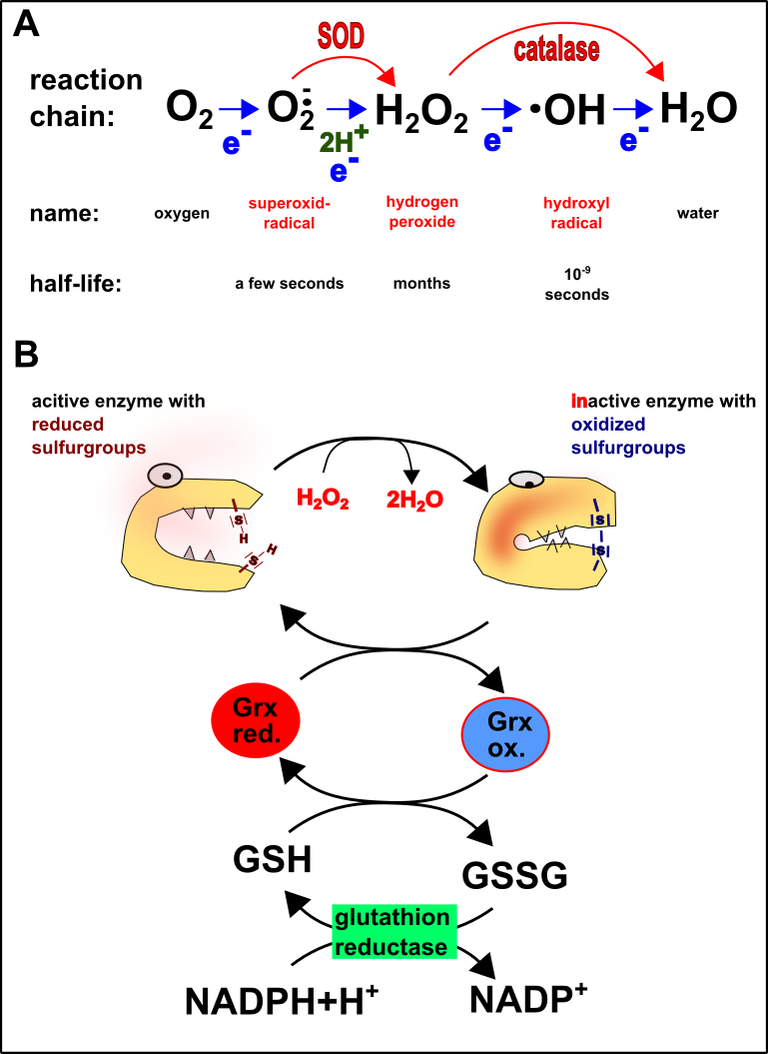
Fig.5 Introduction into redox biology. A. Formation of reactive oxygen species (blue arrows), as well as their degradation by superoxide dismutase (SOD) and catalase (red arrows). B. Inactivation of proteins by reactive oxygen species and rescue by the glutathione system. Grx. = Glutaredoxin; GSH = reduced glutathione; GSSG = oxidized glutathione; NADPH (see [here]). Made by Chapper - unrestricted use allowed
Another active field of the glutathione system is the deactivation of lipid peroxyl radicals. Lipidperoxyl radicals are formed after the reaction of ROS (especially hydroxyl radicals) with membrane components (fatty acids) [19]. Immediately after being formed the lipid peroxyl radicals interacting with multiple cellular molecules. They are further quite stable and reactive. Lipidperoxyl radicals, therefore, harming the cell long-lasting and with high intensity. They can be considered as some kind of cellular nightmare! But no worries: Vitamin E, C and the glutathione system having a plan. At first, vitamin E within the membrane catches one electron of the lipid peroxyl radicals producing a lipid peroxide and a vitamin E radical. Now vitamin C takes over, turning the vitamin E radical back into vitamin E and becoming an ascorbic acid radical (vitamin C radical if you want). Eventually, the glutathione system clears the entire situation by turning the ascorbic acid radical back into ascorbic acid (or vitamin C). Reduced glutathione (GSH) is oxidized in this situation to GSSG which is further reduced back into GSH by the Glutathione reductase (NADPH is needed again) [19].
Indeed, I could move on in this manner and write 300 pages article. For instance, just remember the oxidized guanosine, 8-oxoguanine, which can be found in the DNA of stressed cells. Modifications like them might cause severe DNA damage and mutations (see here and here). Or remember the mechanisms of autophagy, which are responsible to remove harmful mitochondria. Nevertheless, I stop our little journey right here.
Our take-home message for the moment is: High ATP contents are paid with high prices for detoxification and repairing mechanisms.
Nevertheless, billions of years of evolution have passed these tests. It seems like, that flexible nutrient supply, constant cellular conditions plus additional benefits are worth the high efforts the cell has to take.
Oh, while we are already talking about additional services of mitochondria, I have remembered that we forgot to talk about a very special feature. In fact, the mitochondria are serving as some kind of „reset button“. After the cell has exhausted themselves on the hunt for energy, power, and endless growth, the mitochondria stop this „insanity“ by triggering the mechanism of controlled cell death, apoptosis [13, 26, 27]. And this is no seldom happening. Even at this moment millions of cells in your body are killed by apoptosis ([13]: alone 1000000000000 cells in your blood per day!).
Moreover, you have to take into consideration that a cell is not alone, as it was the case in the primordial soup. Therefore, apoptosis is not just a mechanism to remove crazy and dangerous cells, apoptosis is the underlying program for development and order. For instance, in the embryonic neuronal system about half of all neurons getting killed [13]. And this is absolutely important to create an ordered and thus working neuronal network.
Therefore, besides the energetic background, you can see the mitochondria as the basic organelles for the creation of ordered higher organisms.
I think the reader can estimate that mitochondria can be considered as the hidden central within the cell (besides the nucleus). Because of this, it is important to shade more light on them, especially in terms of health and well-being.
But how do they really influence our body and mind? This will be discussed in the next chapters.
So far all the best to you and your mitochondria
Regards
Chapper
May Da Mitos Be With You.

References:
- Wallace, D.C., Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen, 2010. 51(5): p. 440-50.
- Suomalainen, A. and B.J. Battersby, Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol, 2018. 19(2): p. 77-92.
- Hill, B.G., et al., Integration of cellular bioenergetics with mitochondrial quality control and autophagy.Biol Chem, 2012. 393(12): p. 1485-1512.
- W. Janning, E.K., Genetik: Allgemeine Genetik - Molekulare Genetik - Entwicklungsgenetik. 2004: Georg Thieme Verlag.
- Fuchs, Allgemeine Mikrobiologie. Vol. 8. Auflage. 2007: Georg Thieme Verlag.
- Horvath, S.E. and G. Daum, Lipids of mitochondria. Prog Lipid Res, 2013. 52(4): p. 590-614.
- Mileykovskaya, E. and W. Dowhan, Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta, 2009. 1788(10): p. 2084-91.
- Taanman, J.W., The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta, 1999. 1410(2): p. 103-23.
- Koc, E.C. and L.L. Spremulli, Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs. J Biol Chem, 2002. 277(38): p. 35541-9.
- Greber, B.J. and N. Ban, Structure and Function of the Mitochondrial Ribosome. Annu Rev Biochem, 2016. 85: p. 103-32.
- Embley, T.M. and W. Martin, Eukaryotic evolution, changes and challenges. Nature, 2006. 440(7084): p. 623-30.
- Katsura, K., et al., Asaia siamensis sp. nov., an acetic acid bacterium in the alpha-proteobacteria.Int J Syst Evol Microbiol, 2001. 51(Pt 2): p. 559-63.
- Müller-Esterl, W., Biochemie: Eine Einführung für Mediziner und Naturwissenschaftler. 2004: Spektrum Akademischer Verlag.
- Winter, R. and F. Noll, Methoden der Biophysikalischen Chemie. 1998, Stuttgart: Teubner Studienbücher.
- Peter Karlson, D.D., Jan Koolman, Georg Fuchs, Wolfgang Gerok, Ruth Hammelehle, Karlsons Biochemie und Pathobiochemie. Vol. Auflage: 15. 2005: Thieme.
- Brand, M.D. and D.G. Nicholls, Assessing mitochondrial dysfunction in cells. Biochem J, 2011. 435(2): p. 297-312.
- Püschel, Taschenlehrbuch Biochemie. 2011: Thieme Verlagsgruppe.
- al., G.e., Taschenlehrbuch Physiologie. 2. Auflage ed. 2015: Thieme.
- Kalyanaraman, B., Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol, 2013. 1(1): p. 244-57.
- Michalak, K., et al., Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxid Med Cell Longev, 2017. 2017: p. 8023935.
- Brigelius-Flohe, R. and M. Maiorino, Glutathione peroxidases. Biochim Biophys Acta, 2013. 1830(5): p. 3289-303.
- Lopez-Mirabal, H.R. and J.R. Winther, Redox characteristics of the eukaryotic cytosol. Biochim Biophys Acta, 2008. 1783(4): p. 629-40.
- Mari, M., et al., Redox control of liver function in health and disease. Antioxid Redox Signal, 2010. 12(11): p. 1295-331.
- Petrou, M., et al., Amyloid deposition in Parkinson's disease and cognitive impairment: a systematic review. Mov Disord, 2015. 30(7): p. 928-35.
- Wyatt, A.R., et al., Extracellular chaperones and proteostasis. Annu Rev Biochem, 2013. 82: p. 295-322.
- Vanden Berghe, T., et al., Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol, 2014. 15(2): p. 135-47.
- Elmore, S., Apoptosis: a review of programmed cell death. Toxicol Pathol, 2007. 35(4): p. 495-516.
Posted from my blog with SteemPress : http://worldofchapper.de/rp295320-ovh/index.php/2019/08/01/mitochondrial-medicine-fact-or-fiction-chapter-i-mitochondria-our-best-friends/
Scheint interessant zu sein, aber mein Englisch ist so am Boden wie Estrich,dass ich da jetzt nicht viel verstanden habe. Außerdem sind da so viele Fachbegriffe, da müsste ich ja zuerst mal 30 Bücher mindestens lesen, um annähernd zu begreifen, worum es da wirklich geht. Trotzdem schicke ich dir ein kleines upvote per @tipu vorbei. 😉
Posted using Partiko Android
Wow @thales7 vielen vielen Dank!
Es ist natürlich in meinem Interesse möglichst viele Leute zu erreichen. Deshalb werde ich auch meine alten Posts demnächst hochladen (über Wordpress), um noch wesentlich mehr Leute zu erreichen. Aber auch um eine Blogging-Plattform zu haben, wo alles etwas geordneter ist als hier!
Du kannst gern eine Liste von Wörtern oder Inhalten zusammenstellen, welche du nicht verstanden hast. Dann kann ich diese nochmal näher erklären und weiß in Zukunft wo es Probleme geben könnte.
Vielen Dank jedenfalls, dass du es dir angeschaut und so großzügig bereichert hast
Beste Grüße
Chapper
This post is supported by $0.32 @tipU upvote funded by @thales7 :)
@tipU voting service: instant, profitable upvotes + profit sharing tokens | For investors.
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvoting this reply.
Cool thanks!
Wow! Sehr ausführlich und ich muss es mir erst übersetzen und bestimmt mehrmals lesen ;-) LGG
Viel Vergnügen, es auch schon längst ein Video drüber fällig.
Schaffe es nur irgendwie nicht den Schnitt mal fertig zu stellen!
Gruß
Mir reichen die Infos jetzt schon ;-) aber vielleicht ist ein Video einfacher zu verstehen?
Hihi, heißen die wirklich so in Englisch? Wirkt wie ein mystisches Land ;-))))) LGG
Mystik trifft es sehr gut. Ich untersuche die Dinger ja aus beruflichen Gründen jeden Tag und bin immer wieder überrascht. Hab ein schönes Wochenende. Grüße Chapper
Posted using Partiko Android
Dear Chapper,
Thank you very much for helping to explain the mystery of life 🙂 It's almost epic. From the beginning, when bacteria and cells formed a cooperative relationship, to the present where mitochondria have become directors of cell function: the activities of cells controlled by organelles that were once bacteria. Reads like science fiction.
A question about vitamin C: was Linus Pauling's work related to the conversions of radicals (as you describe in your article)?
One function of mitochondria really caught my attention: the role in promoting apoptosis. I know that a failure to clear apoptotic cells is implicated in lupus inflammation. Would this be considered a mitochondrial malfunction?
Wonderful that you take the time to explain all of this to us. I know your research is geared to improving health and you want to share what you know so we can help ourselves. I appreciate that very much.
Thank you. Have the most wonderful summer. Be well, and, as always--don't work too hard.
Regards, AG 🙂
Resteem!
Hey @agmoore,
thanks for your nice comment again.
Linus Pauling's work was even more than that but yes he knew that vitamin C counteracts free radicals and therefore took a few grams a day orally.
In my opinion, it would! We will discuss this in detail in chapter II.
I also wish you a wonderful summer. This year it is not that dry as last year here in Germany, I hope it's the same in the US.
Best regards
Chapper
Thank you! I look forward to that :)
And no, it is not dry. I planted a baby tree and it needs water. I'm so glad.
Enjoy every sunny day... even the rainy ones.
Regards,
AG
I love rain!
But sun is also good 😁
Also a nice Weekend to you.
Posted using Partiko Android
Your personal rain GIF :))
And now it rain outside! Will have a nap to enjoy this.only the rainforest is missing here. Have a nice Weekend. Chapper
Posted using Partiko Android
Thanks a lot. Very interesting!
Thanks! To write this was a pleasure 😉
Posted using Partiko Android
Hi, @chappertron!
You just got a 0.26% upvote from SteemPlus!
To get higher upvotes, earn more SteemPlus Points (SPP). On your Steemit wallet, check your SPP balance and click on "How to earn SPP?" to find out all the ways to earn.
If you're not using SteemPlus yet, please check our last posts in here to see the many ways in which SteemPlus can improve your Steem experience on Steemit and Busy.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Please consider using the steemstem.io app to get a stronger support.
Thank you guys!
Have you read the book 'Head strong' by Dave Asprey? It is very similar to your series I believe, also about how these mitochondria play a crucial role in your body and it proposes ways to improve your mental and physical fitness by optimizing these powerhouses.
I did not really like this authors style of writing. He sounds almost like a Guru who has figured out the one and only way to achieve mental and physical fitness and is eager to share this with anyone. I'm also critical about his arguments because he makes some crucial reasoning mistakes. So I'd love to follow your series to read about the actual science behind it!
Hey @samve,
haven't heard about the book or author before. But I can understand that you are a bit concerned about the style people like he might use. This is often far from the real scientific background and 100% marketing. My job is to do molecular biological and genetical analyses of patients. Therefore, I often wonder about the way these issues are communicated.
First rule: "People are no mitochondria on two legs!"
There is much more you have to consider. Unfortunately, many people reduce complex problems on single origins. That's why I make this series. To explain that a biological system is more complex than the beliefs of "gurus".
Have a nice weekend
Chapper
You could indeed read that there is some truth to what he tells, but it his goal is to convince people rather than giving just the facts. You're doing great in communicating the actual facts so I am very happy to follow your series.
Posted using Partiko Android
With pleasure ;-)
First, i appreciate your way of "teaching' - many infos in clear sentences combined with pictures and humor! If I had a teacher like you at school I would have had much more fun in learning :-) !!!!
This is a little bit like the "stirb und werde" for our life ;-) I like mitochondria! LG Kadna
Posted using Partiko Android
Hey @kadna, I completely forgot to reply. Yes, you understood this completely. It is like a 1 or 0 in computer science. In summary, you have a complex entity: humans!
At the University I was responsible for the molecular biology classes and one day a student comes to me and told me that among the students they said: "All biochemists learning molecular biology from me!" Huge honor when you take into account that they had professors for this!
Have a nice weekend
Chapper
So there is another analogy! Life is a miracle ( Leben ist ein Wunder und wunderbar) Computers, Mitochondrias and human beings...
What has a professor that you don't have?! A piece of paper... and you have much more than he, you listen to your heart! Nice weekend for you!
Posted using Partiko Android
Very nice post! I am looking forward to read the rest (but please take your time as I may not be able to find the time to read 25 posts per week ;) ).
I have a couple of naive questions. First, what do ATP and ADP stand for? Moreover, where is actually the other 10% of the cell energy coming from?
Talking about Alzheimer, I have recently heard at the radio abouta potential way of diagnosing it up to 20 years before the first symptom. have you heard anything about that? Is it related to mitochondria? Sorry for not having any source here, I just heard that at the radio and I did not get any chance to dig further by myself (which is why I ask).
Hey @lemouth, thanks for coming by.
I can understand. I suffer from serious chronic time lack at the moment. You probably recognized this on my curation activity.
Therefore: No worries!
I described ATP in one of my earlier posts in more detail.. Basically, it is one of the components of DNA (A, T, C, G) which also serves as the universal energy carrier. The full-name is adenosinetriphosphate. Mitochondria are responsible for up to 90%, the remaining 10% is from glycolysis or alternative pathways such as nucleotide-kinases or as by-products from various other pathways (but only little amounts). Moreover, you have GTP aside from ATP.
AD is a complex disease and for some cases, you are able to make a prediagnosis since many years (such as for carriers of a certain ApoE allele e4). There are also other risk factors, clusterin (ApoJ) for instance. And there are numerous other molecules (proteases or receptors). When I proceed I could write an entire article about this subject.
And yes mitochondria are of high importance, because of three main aspects: 1. mitochondria trigger cell death. 2. mitochondria producing oxidative stress. 3. mitochondria adjust the cell metabolism.
All three components are hall-mark of AD: elevated cell death, lack of energy, stress!
Because of the breakthrough I also heard something, but can't give you detailed information. When I have more time left, maybe I will write something about this.
I hope this answers the most urgent questions.
Have a nice weekend
Chapper
Thanks a lot for these answers! That is perfect for now!
PS: no problem for the lack of time... this happens :)
Like your blog bro.