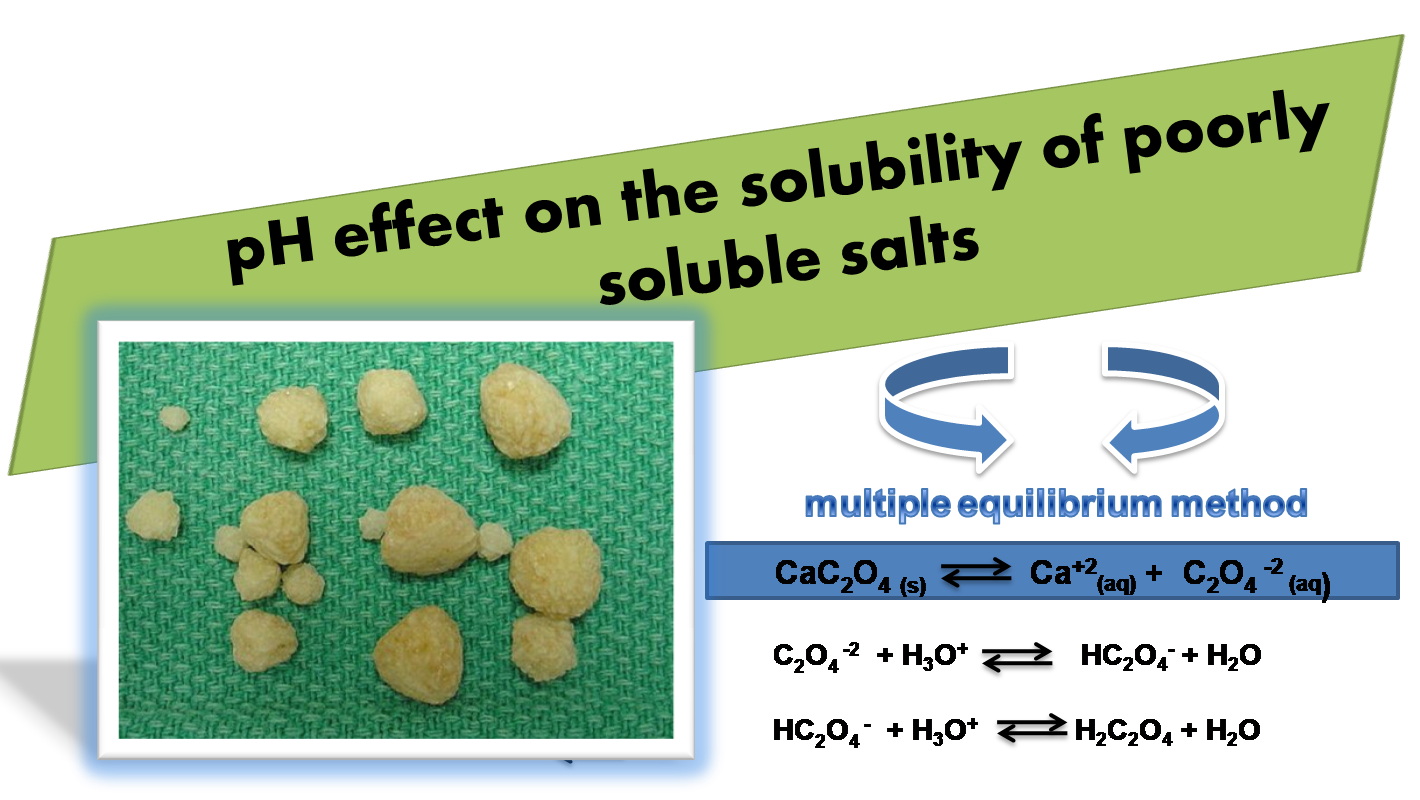
Quantitative study of the influence of pH on the solubility of poorly soluble salts
Source :Image created in power point with public domain image1
Let us analyze its effect from a qualitative point of view
Take for example calcium oxalate, a brown precipitate that is deposited in kegs used in brewing beer and a major component of kidney stones[3]. If a low pH is maintained, the oxalate ion being the conjugate base of oxalic acid will tend to react with hydronium ions to the formation of acid, thus shifting the equilibrium to the right to counteract the disturbance, thus increasing its solubility. This behavior can also be observed in carbonates, sulfides, phosphates.

Effect of pH from the quantitative point of view
On the other hand, it is necessary to consider that other equilibrium are produced as we see in the image in addition to the dissociation of the salt, so it will not be the same to calculate the solubility of a compound omitting these additional equilibrium than to take them into consideration.
In these cases the calculation of the solubility can be a little complicated because of all the equilibrium involved, so we can make use of a method that we will detail below known as Multiple Equilibrium Method.
Multiple equilibrium method
This method consists of developing independent equations that depend on the number of reactants in each particular case, which include the expressions of the equilibrium constant, the mass balance equation and the charge balance equation described below:
A. Expression of the equilibrium constant (Kps): it will depend on the dissociation reaction of each poorly soluble salt and its stoichiometry.
B. Mass balance equation: starting from the information of the solution and the equilibrium established, the concentration of the different species in the equilibrium and their analytical concentration are related.
C. Charge balance equation: since electrolyte solutions are electrically neutral, it can be expressed in the following way
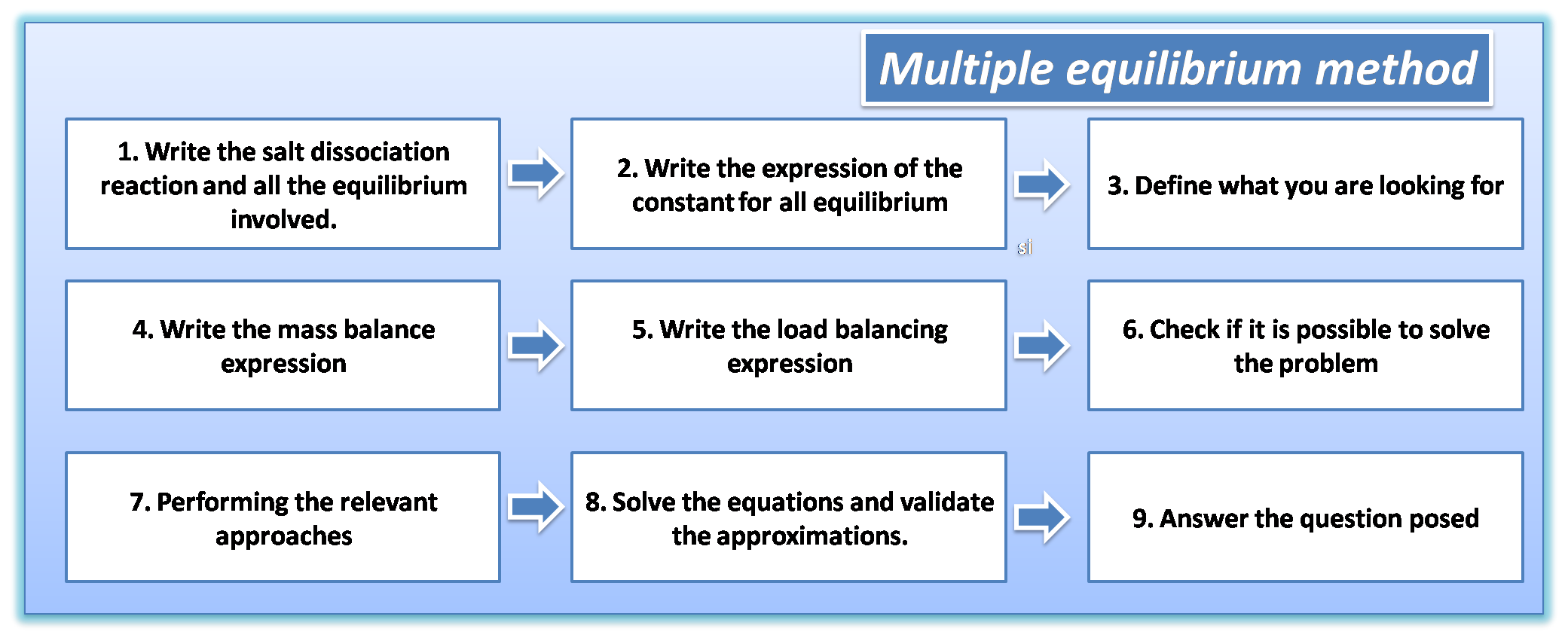
Stages of the multiple equilibrium method
Problems involving several equilibrium can be solved by following the steps shown in the following diagram:
Calculation of the solubility of a poorly soluble salt by applying the multiple equilibrium method.
Consider, for example, the case of calcium oxalate, and calculate the solubility in a solution with a constant pH of 4.00 and whose [H3O+] is equal 1* 10-4.
Part A
The solubility of the salt in pure water is calculated, without taking into account the different equilibrium occurring in the solution as follows and the results can be compared.
- Dissociation of the salt
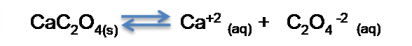
Expression of the solubility product constant
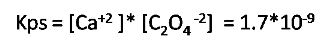
Calculation of the solubility from the Kps value
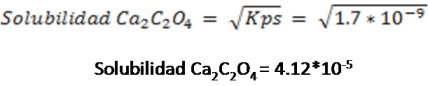
PART B
The solubility of the salt is calculated following the steps of the method
1: Write the dissociation reaction of the salt and all the equilibrium involved.
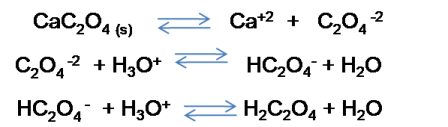
2: Write the expression of the constants for all equilibrium with their tabulated values respectively according to the literature consulted.
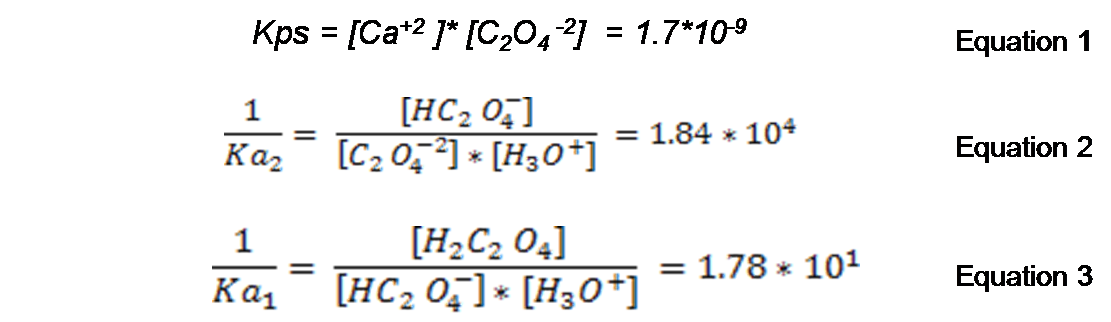
3: Define what is sought in terms of equilibrium concentration. In this case the concentration of calcium oxalate is equal to the analytical molar concentration of calcium ion, and is expressed as:
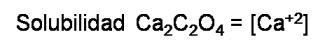
4: Write the mass balance expression: As we see in the equilibrium, the only source that provides us with calcium is the dissociation of calcium oxalate and stoichiometrically is equal to the oxalate concentration.
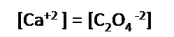
However, since oxalate participates in other reactions the expression is written as.
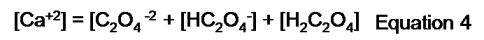
5: Write the charge balance expression: In this case, we are told that the solution is maintained with a pH of 4.00; however, the species is not detailed, so we do not have the information required to perform the charge balance.
6: Verify if it is possible to solve the problem: At this point we must verify the number of unknowns and equations to know if a solution can be obtained. If we count we can see that there are 4 unknowns presented in the mass balance expression and there are 4 equations so we can continue solving the problem.
7: To make the pertinent approximations. In this case it is not necessary to make approximations since it can be solved directly.
8: Solve the equations.
we can clear from equation 1 [C2O4 -2].
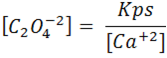
From equation 2, we can clear [HC2O4-]
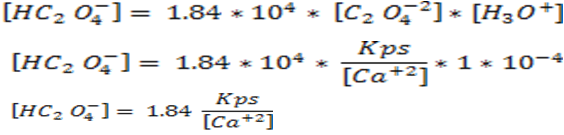
and from equation 3 we can clear [H2C2O4]
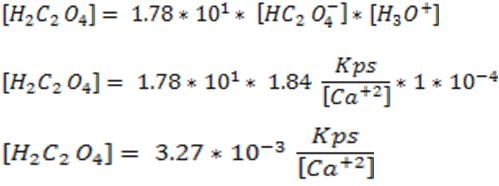
Substitute the concentrations [C2O4 -2] , [HC2O4-], [H2C2O4] in equation 4.
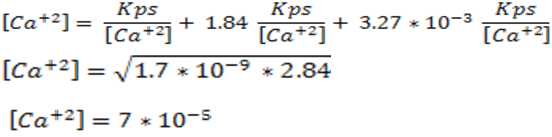
9: Answer the question posed
Analysis
As can be observed, the solubility of calcium oxalate in a pure water solution is 4.12* 10-5 M, however, when the pH of the solution was lowered to 4.00 and taking into account all the equilibrium involved, the solubility of the salt increased to 7*10-5 M, which shows the displacement of the equilibrium.
It is worth noting the importance of the application of the multiple equilibrium method for the calculation of this type of compounds since all the equilibrium and species involved are taken into account and a more accurate result is obtained.
I hope you find the information I have presented here useful, until next time!
References
[1]Skoog, W. (2000). Analytical Chemistry. Mc Graw Hill
[2] Harvey, D.(2002). Modern Analytical Chemistry. Mc Graw Hill.
[3]Calcium oxalate. Available at:
Very useful information, especially for someone like me who studied analytical chemistry years ago and started to forget it lol, thank you
Hello @benainouna!
The study of the factors that affect the solubility of this type of compounds is important to know what modifications can be made at laboratory and industrial level. Thank you for your comment.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.