Potentiometric analysis
Greetings dear friends of Hive. This time I bring you information related to potentiometry and its applications. I invite you to check it out!

source: @yusvelasquez
Potentiometry is the most widely used electrochemical interface method in analytical applications. It measures the potential of an electrochemical cell under static conditions, since no or a negligible amount of current passes through the solution while its potential is measured[1].
The first quantitative applications of potentiometry appeared soon after the Nernst equation was formulated in 1989, which relates the potential of an electrochemical cell to the concentration of chemical species present in the cell[2]. Although at that time potentiometry was limited to redox equilibria at metal electrodes, so it was only applicable to a few ions, but in 1906 when it was discovered that between two opposite sides of a thin glass membrane in contact with solutions containing different concentrations of H+ ions a potential difference is established, it led to the development of glass pH electrodes, which was followed by other types of membranes with useful applications[1]. Thus, the continuous development of electrodes has allowed the use of potentiometry to be expanded.
Potentiometric measurements
In order to carry out a potentiometric analysis, it is essential to have a cell and two electrodes. Measurements are carried out with a potentiometer, determining the potential difference between an indicator or working electrode and a reference electrode. And since no significant current flows through the potentiometer, the mission of the reference electrode is to supply a potential that precisely serves as a reference.
The following image represents the typical schematic diagram of a potentiometric electrochemical cell.
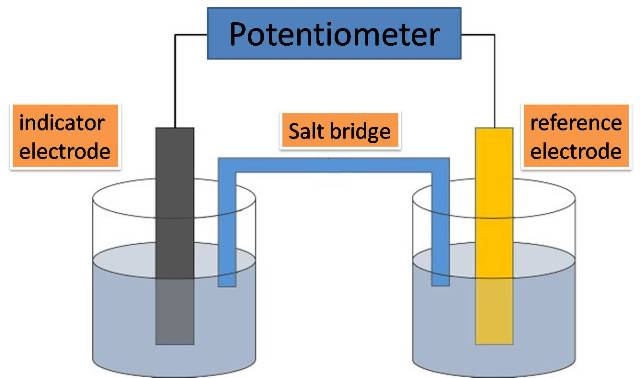
Figure 2. General representation of the components of an electrochemical cell for potentiometry. Source: @yusvelasquez.
As we can see the cell is divided into two halves, each of them with an electrode immersed in a solution whose concentration depends on the electrode potential. In the half of the cell where the indicator electrode is located, there is the solution whose concentration is to be measured, while in the other half we have the reference electrode, immersed in a solution whose concentration is known.
The separation of the electrodes is necessary to avoid a spontaneous redox reaction on the surface of one of the electrodes. And the two halves are connected by a salt bridge, which is basically an inert electrode, usually KCl, which allows the displacement of ions between the two half-cells, thus completing the electrical circuit.
Electrodes
Potentiometric cells are constructed in such a way that one of the half-cells provides a reference potential and the potential of the other indicates the concentration of the analyte. For convenience it has been adopted that the reference electrode is the anode, and is denoted tachographically as:
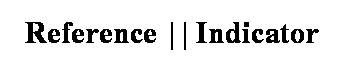
Reference electrodes and their characteristics
An ideal reference electrode should have a power that is accurately known and keep it constant with the passage of current, and should be insensitive to the composition of the analyte[3]. Some of the commonly used reference electrodes are:
Normal hydrogen electrode
Although it is rarely used in routine analytical work, it is one of the most important because it is used to establish the standard potentials of the other half-reactions. It is based on the reduction of the H+(ac) ion to H2(g) at a platinum electrode, the reaction is:
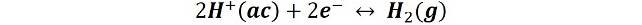
And by definition the standard potential of the reaction is 0.0 V at any temperature.Calomel electrodes.
These are based on the redox couple formed by Hg2Cl2 and Hg, the reaction is:
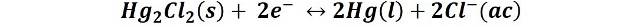
The saturated calomel electrode which is made from a saturated solution of KCl is the most widely used because it is very easily prepared, its electrode potential of this electrode is 0.2444 V at 25 °C.
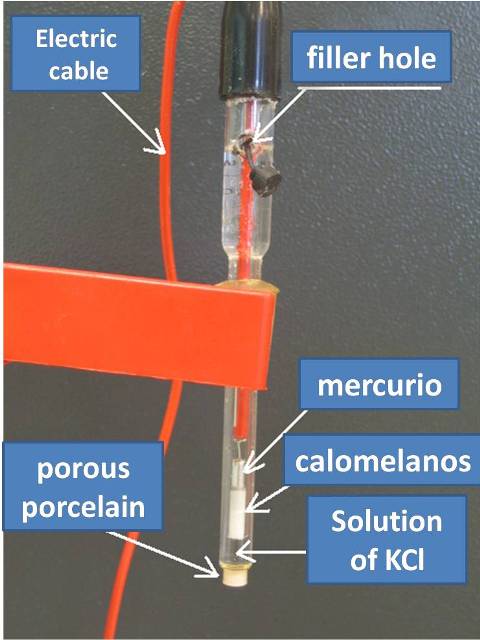
Figure 3. Saturated calomel reference electrode. Source: Alain Le Rille (2010), on Wikipedia.com, CC BY 2.5.
Indicator electrodes and their characteristics
The most commonly used are:
- Metallic electrodes.
This type of electrodes is convenient to classify them as first species, second species and inert redox electrodes. First species electrodes are formed by a pure metal that is in equilibrium with its cation in solution, conducting electrons towards the potentiometer. Some of these are: Ag, Zn, Cu, Hg, Sn, Tl, Cd and Bi.
While the electrodes of the second species are also metallic, they not only respond to their own cations but are also sensitive to the activity of anions that form precipitates or complexes with these cations.
- Membrane electrodes
The most notable example of this type is the pH meter, which relies on glass membrane electrodes as meters of the concentration, or to be more precise the activity, of H+ ions in solution.
These perform a measurement of the potential generated across a thin glass membrane separating two solutions having a different concentration of H+ ions.
Glass pH electrode
This consists of a glass indicator electrode and a saturated calomel electrode as a reference electrode[3]. The indicator electrode consists of a thin pH-sensitive glass membrane at the end of a thin plastic tube, inside the tube is a small volume of dilute hydrochloric acid in saturated silver chloride solution, and inside this solution is a silver wire which acts as the reference electrode.
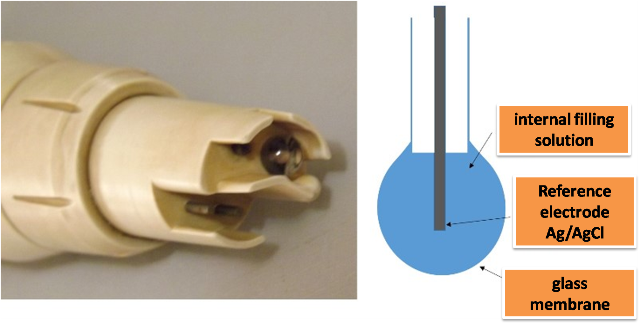
Figure 4. Glass electrode for pH measurement. Source: @yusvelasquez.
Glass electrodes for cations
Some studies on the composition of the glass membrane have led to the design of electrodes that can measure ions other than hydronium, developing electrodes to make direct measurements of species such as Na+, K+, Li+ and Ag+ and NH4+.
Potentiometric calculations
Potential and concentration
The potential determined in potentiometric analysis is that of a cell, which is given by:
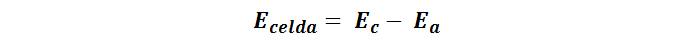
Where Ec and Ea are the reduction potentials of the reactions occurring at the cathode and anode. And these potentials are related to the concentration of the analyte by the Nernst equation:
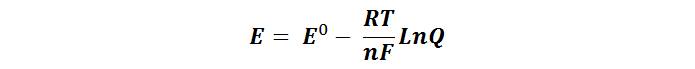
Where E0 is the reduction potential at standard conditions (these are tabulated for different reduction reactions), R is the gas constant, T is the absolute temperature in Kelvin, n is the number of electrons transferred, F is Faraday's constant and Q is the corresponding reaction quotient.
Applications
The potentiometric determination of an analyte is a very frequent operation in a laboratory, perhaps the most common is the measurement of the pH of a solution, but potentiometric titrations are also a widely used tool in analytical chemistry and physicochemistry for the titration of species. This does not leave out other areas in which potentiometric measurements are important, for example, specific electrodes have been developed for the determination of Zn(II) in biological, environmental and medicinal plant samples, as well as for the determination of phosphate and ammonium ion in pharmacology and environmental chemistry[4].
In conclusion, potentiometry as an analytical technique has multiple applications, especially for monitoring the concentration of ions in water samples and various electrolytes in biological fluids, which makes it a technique of easy application in several areas of study.
Well friends, this is the end of this post, I hope you find the information presented here very useful, see you next time!
References
- Harvey, D. (2002). Modern analytical chemistry. McGraw-Hill.
- Wikipedia.com. Nernst equation.
- Skoog, West, Holler (2001). Analytical chemistry. Seventh edition, McGraw-Hill.
- Trujillo, A., Vega, P., Barajas, L. (2014). Potentiometry: uses and applications.
Your content has been voted as a part of Encouragement program. Keep up the good work!
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for Proposal
Delegate HP and earn more
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Hi @yusvelasquez your dissertation has enough to understand about potentiometric analysis. Particularly, I accompanied researchers from a Chemistry Research Center in developing pilot instruments to do their tests, one of them was to measure pH. Thanks for sharing.
Hello @alfonsoalfonsi!
What a good experience, these instruments are widely used at the laboratory level in many analyses and it is important to know how they work. Thanks for commenting!