polyprotic acids
Hello dear friends!
These compounds can be obtained by means of different industrial processes and serve as raw materials for the production of other substances, as well as being very common in nature and playing a fundamental role in living beings.
Their classification depends on the number of protons per molecule that they can yield in a chemical reaction. For example, hydrochloric acid (HCl), acetic acid (CH3COOH), hydrocyanic acid (HCN) are called monoprotic acids because they contain only one ionizable hydrogen. Dissociation of acetic acid:

Among the diprotic acids we can mention sulfuric acid (H2SO4), carbonic acid (H2CO3), hydrogen sulfide (H2S) since they contain two ionizable hydrogens per molecule. Polyprotic acids are considered to be those containing two or more ionizable protons. The main difference between a monoprotic acid and a polyprotic acid is that the latter gives up several protons and does so in several stages of deprotonation.
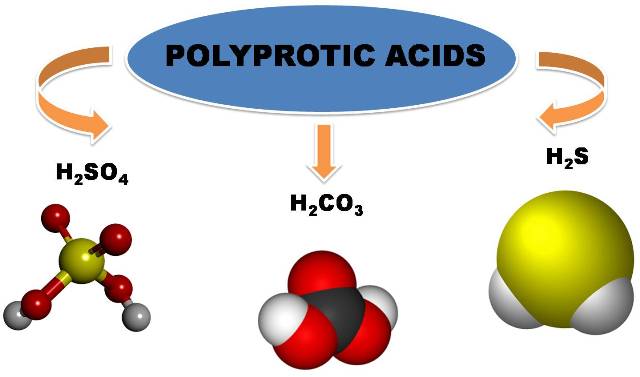
Image made in power point with public domain images 1,2,3
Let us analyze the polyprotic acids!
Let us take as an example carbonic acid, with molecular formula H2CO3. This acid has multiple uses at the industrial level as well as for human consumption. It is used in the manufacture of carbonated beverages, energy drinks, beers, for the production of bubbles, in the elaboration of effervescent tablets such as vitamins. It is also used in the preparation of soil for crops as it optimizes plant growth through its pH regulating effect, accelerates the curdling process in the dairy industry, among others.
Carbonic acid dissociation
Carbonic acid H2CO3 loses a proton to form HCO3- and then this ion gives up the other proton to give rise to CO3-2.
Dissociation is given by the following reactions.
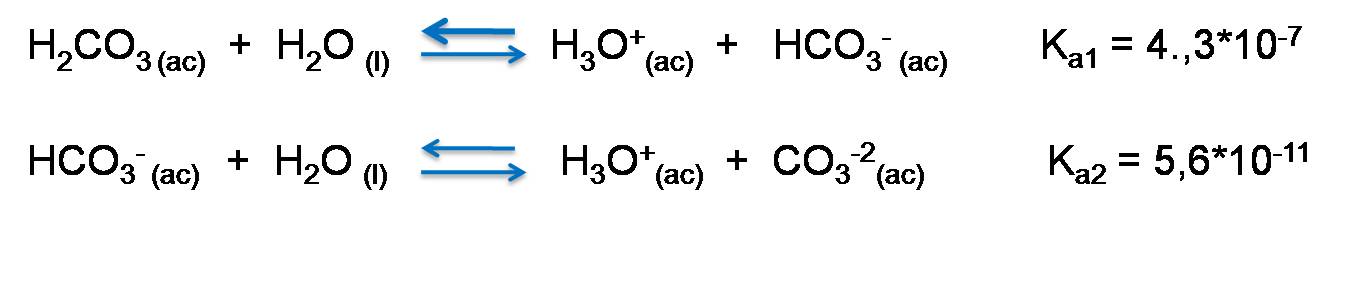
Equilibrium constant of polyprotic acids.
As we can see, carbonic acid gives up its protons consecutively, and the value of the equilibrium constant is decreasing significantly, usually by a factor of about 103, so that
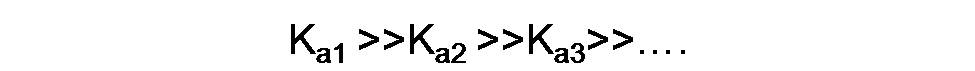
In the first dissociation its value is 4.3*10-7 while in the second it decreases to 5.6 *10-11, so the second reaction occurs to a lesser extent.
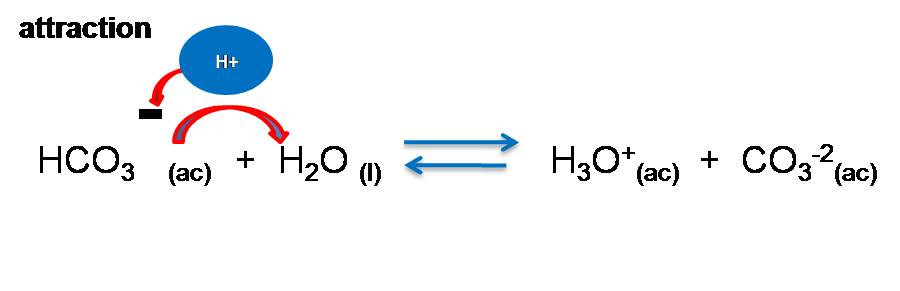
What is the reason for this decrease?
It is due to the attraction of electrostatic forces between the ions, in the first dissociation reaction the negatively charged HCO3-- is formed, when it is going to yield the proton to the water molecule, there is a strong attraction with the CO3-2 ion that prevents it from yielding as easily as H2CO3 did and therefore the reaction is carried out to a lesser extent.
Here are some examples of polyprotic acids and the value of their acidity constants
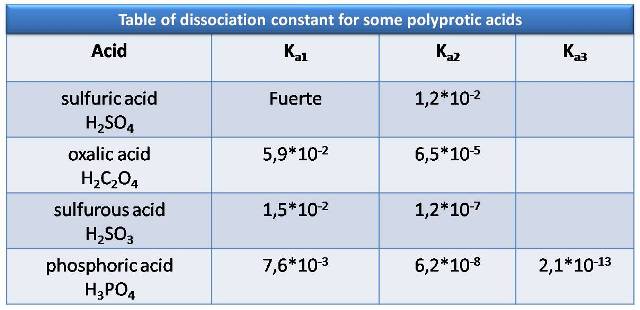
Source: Atkins, P. (2006)
Calculation of pH of a polyprotic acid
Polyprotic acids are mostly weak, with the exception of sulfuric acid which is a strong acid in its first deprotonation, therefore their dissociation constants are usually widely different. Thus, polyprotic acid can be treated as the only predominant species in solution. Making this approximation leads to a significant simplification in calculating the pH, so that only the value of the first dissociation is used, treating it as a weak monoprotic acid. This does not mean that the rest of the deprotonations do not occur, only that they do not affect significantly and can be ignored for calculation purposes.
Useful tool for pH calculation
Principle: it is assumed that the species in greatest proportion is polyprotic acid, and that only the first deprotonation contributes significantly to the concentration of H3O+ and that autoionization of water does not contribute significantly to the concentration of H3O+ y OH-.
Procedure:
- First step: will continue with the example of carbonic acid using an initial acid concentration of 0.1mol/L.
From the first dissociation, the concentrations of the H3O+ ion and its conjugate base are calculated using the following equilibrium chart
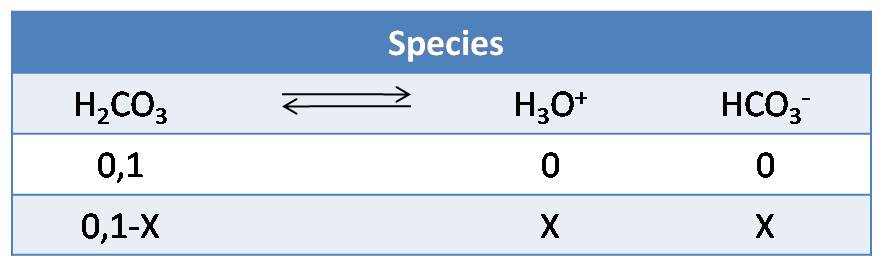
- Second step: Calculations from the first dissociation constant
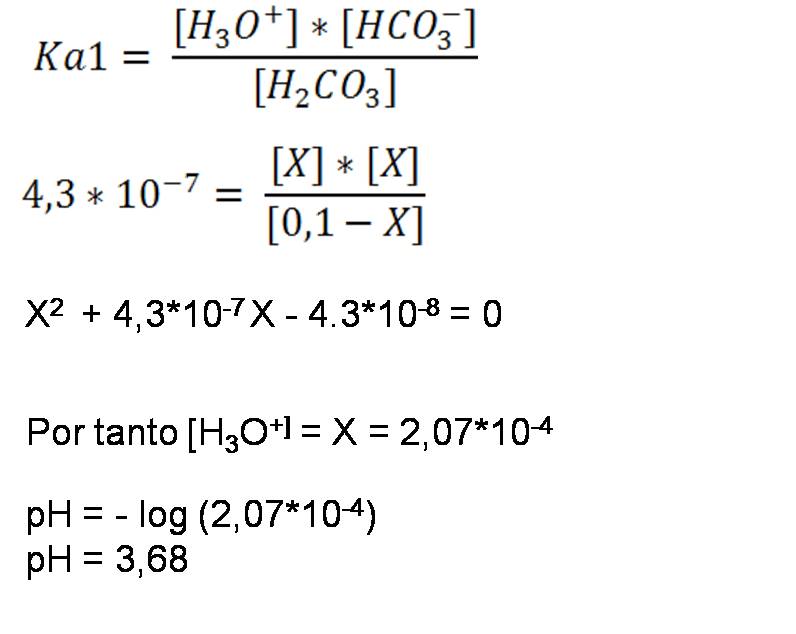
Composition and pH
In some cases it is necessary to have information about the concentration of the different species in a solution of a polyprotic acid and how they vary with pH. For example, if we analyze rainwater that has not been altered by products from human activities, it is mainly made up of weak acids and has a pH of approximately 5.7, the majority being carbonic acid, which results from the dissolution of carbon dioxide from the atmosphere in the water. When its concentration is low it could be expected that at low pH the completely protonated species H2CO3 would predominate, however, if the pH increases the predominant species would be the intermediate species, the HCO3-, and if its value increases one more, then the completely deprotonated species CO3-2 would predominate.
The main pollutants found in acid rain are strong acids that come from human activities.
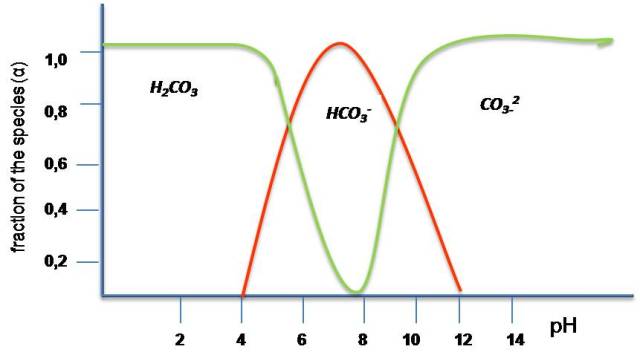
Source: @yusvelasquez.
From what is observed in the graph it is deduced that the presence of the deprotonated species increases as the pH of the solution increases.
Titration of a polyprotic acid
In the titration of this type of compounds several end points are generated depending on the number of protons it possesses, and as long as they have a different acidic strength.
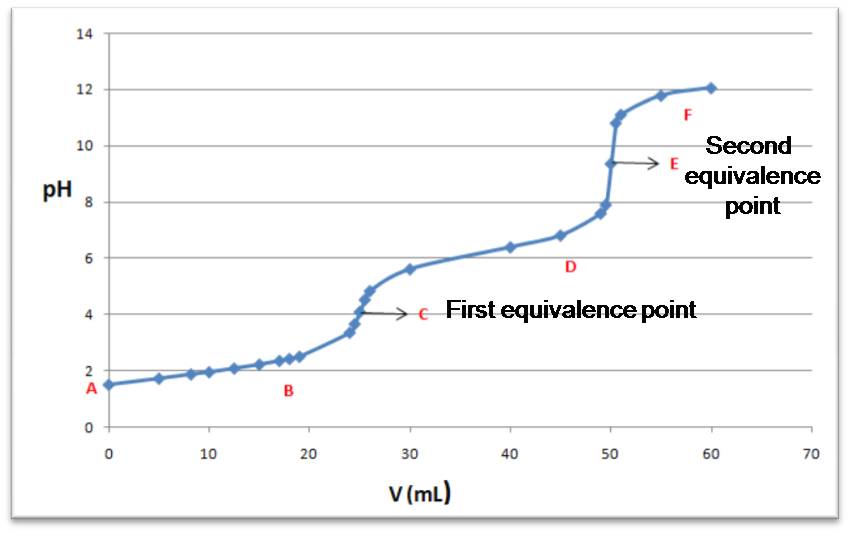
Source: @yusvelasquez.
As can be seen in the figure, which shows the typical titration curve of this type of acids, the initial pH will depend on the acid and it can be studied as a monoprotic acid with only the first dissociation predominating. In zone B, you will have a buffer solution formed by H2A and HA-. At the first equivalence point, an acidic solution formed by HA- will predominate. In zone D a second buffer HA- and A-2 will form. At the second equivalence point the A-2 salt will form, and beyond this point the excess of added base will predominate.
The calculation of pH in each zone for this type of compound is a little more complex than for monoprotic acids and will be detailed later.
In conclusion, we can observe that depending on the type of acid, the dissociation reactions and the hydrogen ions that can be yielded will depend on the type of acid. Their study is important because from these reactions it is possible to calculate the concentrations of the different species in a given solution.
So far for the moment, I hope the information I present to you is very useful!
Bibliographical references
- Whitten K. and Gailey, K. (1985). General Chemistry. Mexico: Nueva Editoral Interamericana
- Atkins, P. and Jones, L. (2006). Principles of Chemistry. The paths of discovery. Buenos Aires: Médica Panamericana
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Congratulations @yusvelasquez! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
Your next target is to reach 45000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOP