Chemical indicators. Function and examples
One of the things that most calls our attention in chemistry are the attractive color changes produced by the reactions, this characteristic is also used to determine the moment in which a chemical change has occurred, being the basis of many methods of analysis.

Methyl orange is an acid-base indicator. Source: @yusvelasquez.
However, in many cases the chemical reactions can occur imperceptibly to the eye, for example, in the neutralization of hydrochloric acid with sodium hydroxide the products of the reaction are sodium chloride (common salt) and water, it is understandable to imagine that both if the reagents and products are colorless, how could we then know when the reaction is complete?
So, in cases like this, in order to follow the reaction and determine the end point of a titration it is necessary to add some other substance that signals to us that the expected chemical change has occurred without this substance interfering with the main reaction; these substances are the so-called chemical indicators.
Chemical indicator
The indicator is a substance that fulfills the function of indicating that a change has occurred in the reaction medium in which it is present; generally the signal is a sudden change in color when another of the reagents has reached a certain concentration.
Specifically speaking, chemical indicators are weak organic acids or bases whose base or conjugated acid pair has a different color due to differences in their absorption spectrum [1]. They are used in very low concentrations so they do not interfere with the reaction of interest that is carried out.
They have complex structures, so to simplify they are represented by the general formula HIn, and their ionization is represented by the following equilibrium:
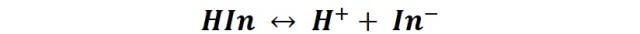
So you can define your equilibrium constant as:
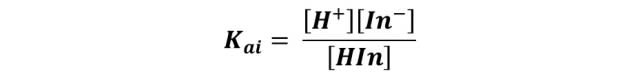
The one we can reorganize to give:
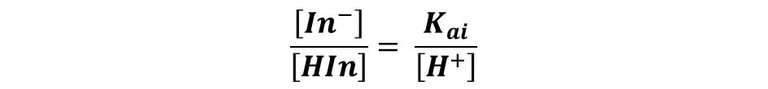
Then, when the concentration of H+ is greater than 10Kai, the color of In- is the dominant one, while the color due to HIn will dominate when the concentration of H+ is less than Kai/10.
What are chemical indicators for?
As I had already mentioned, indicators are used to signal a chemical change that occurs in the reaction in which they participate, but this is necessary for two reasons:
- They give us a reference of a property such as pH or oxidation state.
- They indicate the end point of a titration.

Example of a valuation using phenolphthalein as an indicator. Source: @yusvelasquez.
Types of chemical indicators
There are different types of indicators whose use depends on the reaction in which they are needed. Thus, we have acid-base indicators (whose color varies according to the pH of the solution), oxide-reduction indicators (which depend on the state of oxidation of a substance), precipitation indicators, among others. Let's talk about some of them.
Acid-base indicators
These are perhaps the best known, as they are widely used. They vary in color according to the pH of the solution in which they are found. In general, they are organic molecules that act as a color dye, although most dyes have a single color, the color of these molecules respond to a change in the concentration of hydronium ions in the solution. Most of these indicators are in themselves weak acids or bases, but affect the pH of the solution because they are used in very low concentration and in small amounts, just a few drops are enough to produce a change in color.
The equilibrium reaction of an acid-base indicator can be written as:
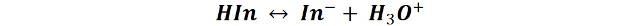
Thus, it can be seen that when the concentration of the acidic substance increases (H3O+) the balance of the reaction shifts to the left, so that the color of the HIn form predominates. While, in the opposite case, when the ion concentration in the middle decreases, the equilibrium moves to the right, predominating the form In-. In this way color changes from 1 to 2 and vice versa occur.
Some indicators have only one color turning point, while others can do so at different intervals. So, properly selected, an indicator gives us a visual reference of the approximate pH of a sample.
Let's take bromothymol blue as an example in three different solutions. When we add the indicator to a test tube with hydrochloric acid solution (pH<6), we will see a yellow color, if we add the same indicator to a test tube with water, we will see a green color (pH=7), and if we add the indicator to a solution with sodium hydroxide (pH>7) we will see that the solution is stained blue.

Color changes of bromothymol blue in acid (yellow), neutral (green) and basic (blue) solutions. Source: @yusvelasquez.
Indicator paper
It is very common in laboratories, and very useful for measuring in a very simple way the different pH in solutions. It is sufficient to introduce one of the pieces of paper into the problem solution, and this immediately reacts by showing a certain color, which is then compared with the scale provided on the package establishing the pH of the solution.

pH indicator paper. Source: Wikimedia Commons, CC-BY-SA-2.5.
Redox indicators
Oxidation-reduction reactions generate electrode potentials that can cause changes in some substances, serving as an indicator for this type of reaction.
Specifically, a redox indicator is a substance that changes color when it changes from its oxidized form to its reduced form[2]. An example of this is 1-10 phenantroline, better known as ferroine, this molecule changes from pale blue to red.

Color of the ferroine according to its oxidation state. Source: @yusvelasquez.
This chemical compound has by formula C36H24FeN6SO4, which in abbreviated form is written as [Fe(o-phen)3]SO 4 denoting 1-10 phenantroline with o-phen; its active ingredient is the ion [Fe(o-phen)3]2+, which is a chromophore that can be oxidized to its ferric state [Fe(o-phen)3]3+.
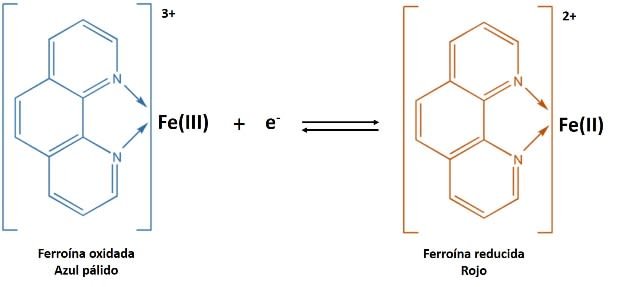
Ferroin color change. Source: image adapted by @yusvelasquez, original source Wikipedia.com, CC0.
Another indicator widely used in oxidation-reduction reactions is methylene blue; in its reduced state it is blue, while in its oxidized state it is colorless.
Precipitation indicators
The use of chemical indicators is one of the simplest ways to determine the end point of a precipitation volumetry. Two types can be distinguished, those that lead to the formation of a colored compound either solid (Mohr method) or in solution (Volhard method), or those that are absorbed over the precipitate at the end point of the titration.
For example, the Mohr method is widely used for the determination of the chloride ion using the silver ion as a titrant:
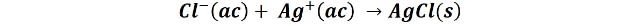
The silver chloride produced by the reaction is obtained in the form of a white precipitate. To determine the end point of the titration, the chromate ion is used, and the end point is determined by the appearance of a red precipitate of Ag2CrO4. Since the solubility of silver chloride is lower than that of silver chromate, the latter will only appear when the silver chloride has precipitated completely, thus indicating the end point of the titration.

Color change in a Mohr titration, the yellow color of the potassium chromate changes to red when the silver chromate is produced. Source: @yusvelasquez.
Metallochromic or complexometric indicators
These are ionochromic indicators, which means that it changes the color of the solution in which it is found in the presence of certain ions and returns to its original state once these ions are removed from the solution[4]. This type of indicator is used in titrations to determine when metal ions are chelated, usually EDTA is used as a chelating agent.
Other indicators widely used in this type of assessment are Erichrome black T, for the determination of calcium, magnesium and zinc ions; Murexide for calcium ions.

Color change of Erychrome black T, it changes from blue to red when Ca2+ ions are present. Source: @yusvelasquez.
Conclusion
Chemical indicators play a decisive role in the titration processes, since they are indispensable for the determination of the end point of the titration and thus to be able to determine the concentration of the analyte in the sample. Basically, any process carried out in the pharmaceutical, cosmetic and food industry requires a strict control of acidity; or for example, the quantification of metal ion numbers in water is essential to know if it is suitable for consumption, and this would not be possible without a simple and effective means to determine these species or chemical property.
This publication provides a simple review of the main types of chemical indicators and their application. Since acid-base indicators are generally better known, the existence of other types is often ignored; moreover, it can serve as a reference for chemistry students so that they know the principle of each type of indicator.
References
- Chemistry Libretexts. Indicator.
- Harris, D. (2001). Análisis químico cuantitativo. Quinta edición, editorial Reverte S.A.
- Wikipedia.com. Ferroína
- Wikipedia.com. Indicador metalcrómico
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app and including @stemsocial as a beneficiary, which give you stronger support.