Acidity in whole milk, where it comes from and how it is determined
We all know that milk is a very important food in our diet, its direct consumption is a source of calcium and other minerals, and it also serves as a base for the preparation of various derived products, such as cheese, butter and yogurt among others; that is why it is essential to obtain and preserve it in the best conditions that guarantee its safety at the time of consumption or use it as raw material to make other products.

Source: @yusvelasquez.
However, to guarantee the quality of this important food, standards and technical requirements have been established to which all milk produced for human consumption must conform. Although, the main regulations emphasize that it is free of foreign particles and pathogenic germs, the physicochemical aspects touch upon the indispensable conditions that are necessary to offer the consumer a quality product. If we take it into account, milk constitutes an ideal medium for the procreation and propagation of diverse microorganisms, among which diverse bacteria can prosper. These bacteria can be classified in five groups, being: coagulant acidifiers, common acidifiers, inerts, alkaline and peptononizers[1].
Among these groups, the acidifying bacteria represent a good proportion of the microorganisms present in milk. Therefore, a measure of the acidity developed in milk is an indirect measure of the bacteria present in it, and is related to the quality of the product. On the other hand, the measure of acidity determines many conditions during the manufacture of derived products, yogurt, cheese and butter.
That is why, among the regulations adopted to guarantee the quality of milk, the determination of acidity is included, besides it is an indispensable control parameter during the elaboration of its derived products.
And where does milk acidity come from?
Immediately after milking, the acidity of the milk is developed mainly by phosphates, caseins and carbon dioxide that are among its main components, then, this parameter is modified by fermentation processes that are attributed to microorganisms of the group Streptococcus lactic, which form lactic acid from lactose. This acidity in fresh milk is around 0.15 to 0.16%, and is expressed as a percentage of lactic acid[2].
Microorganisms form lactic acid from lactose. Source: image edited by @yusvelasquez, original from pxhere.com, CC0.
If during its production, the relevant sanitary conditions are not ensured, high values of acidity can be found due to an increase in the concentration of lactic acid, which is attributed to the contamination of the product with aerobic mesophilic bacteria that ferment lactose. During this fermentation process, the percentage of lactic acid can easily exceed 0.18%, the maximum limit accepted by industrial plants to receive the product, since the acidity character can cause undesirable secondary reactions[2].
How is it determined?
Acidity in whole and sterilized milk is defined as the content of free acids and those associated with weak acids, and is expressed as the content of lactic acid present in a 100 mL sample of milk.
Therefore, it can be determined experimentally by neutralization volumetry, using a standardized basic solution, such as sodium hydroxide, and phenolphthalein as an indicator. Calculating the lactic acid concentration from the balanced neutralization reaction, e.g. to determine the acidity of the milk, proceeds according to the equation:
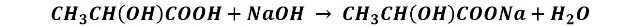
Then, 1 mole of lactic acid reacts with 1 mole of NaOH so the number of moles of lactic acid is equal to the number of moles of NaOH used in the titration:
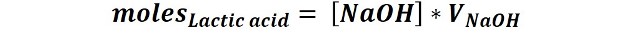
Therefore, once the amount of sample to be titrated, the concentration and volume of NaOH used in the titration has been determined, the acid concentration in the sample can be determined.
Experimental method
The determination of the acidity of the milk is made by means of the titration of aliquots of the product.
Materials and reagents
125 mL Erlenmeyer flask
10 mL volumetric pipettes
50 mL graduated burette
Phenolphthalein solution 1%
Sodium hydroxide 0.1 N
Procedure
- Mix the sample until homogenized and bring to a temperature of 20 °C. If lumps of cream are observed that do not disperse, heat the sample in a water bath at 38 °C until it is homogeneous, then let it rest and cool it down to 20 °C.

Sample preparation. Source: @yusvelasquez.
- Measure 10 mL of the prepared sample with a volumetric pipette and transfer to an Erlenmeyer flask.

Measurement of the aliquot. Source: @yusvelasquez.
Add 5 drops of phenolphthalein.
Titrate with 0.1N sodium hydroxide until a persistent pink color.

titration until the appearance of the pink color. Source: @yusvelasquez.
- If the color persists for 30 seconds, record the spent volume of sodium hydroxide.

Comparison of the initial sample with the end point of the titration. Source: @yusvelasquez.
Expression of results
The results are expressed in mL of NaOH 0.1N/100 mL of milk, or as g of lactic acid/100 g of milk.
The following table shows some average values measured in a milk obtained in the market.
Table n°1. Average acidity in a full milk sample.
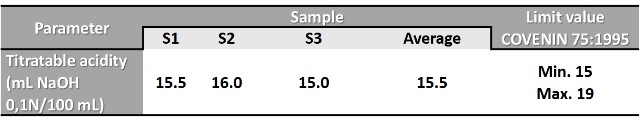
If we want to express the acidity in terms of percentage of lactic acid, we proceed to the following operation.
For a 10 mL sample of pasteurized milk that consumed 1.5 mL of NaOH of 0.103 N concentration, we proceed:
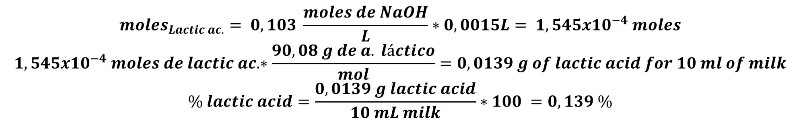
This form of expression is also important, since some international standards such as UNE 34-100, set the maximum limit of acidity in milk at 0.2% in lactic acid.
Conclusion and contribution
As we have seen, the acidity of the milk is a very important physicochemical parameter to control the quality of this product, which, due to its importance in our diet, especially that of children, it is essential to guarantee its quality and the safety of the consumers.
The volumetric method is one of the most used methods to determine the acidity of milk, although there are other methods that can be used to determine the lactic acid content of milk, such as spectrophometric and chromatography techniques, many times these procedures are not available to small analysis laboratories, especially if we are talking about small or medium production units, which make up a large part of the agro-industrial sector of Latin American countries. Therefore, the knowledge of this method by the student and the professional of chemical analysis is a reliable and accessible way to assure the quality of the product.
Well friends, until here the present post, I hope you liked the information presented. Remember the importance of analytical methods in food quality control. See you next time!
References
- Ramirez, G. Una prueba práctica para la acidez de la leche. document online
- Chacón, A. (2006). Comparación de la titulación de la acidez de leche caprina y bovina con hidróxido de sodio y cal común saturada. Agronomía Mesoamericana 17(1) – pag. 55-61.
- Norma COVENIN 658-1997. Leche y sus derivados. Determinación de la acidez titulable.
- Norma COVENIN 75:1995. Leche pasteurizada. 2da revisión.
Congratulations @yusvelasquez! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Hello @yusvelasquez,
An interesting article. Some people boil milk just before eating it, to make the milk more digestible. Does this reduce the acid content?
Thanks!
Greetings @agmoore, I'm glad you liked the article. I don't think that by boiling the milk its acid content decreases, by subjecting it to a thermal treatment, similar to pasteurization, the lactose and other mineral content is not lost, and that is what causes its intolerance. And thanks to you for coming to read.
Thank you!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Thank you my friends!