How The Sun Really Shines - No Light Without A Tunnel!
Since ancient times, humans have realized how important the sun was to their existence. Rightly so, for without it, none of us or any form of biological life could have formed on this big ball we live on which we call Earth.
The sun has been worshiped as a diety by many ancient civilizations, our calendar revolves around our planet's trip around it and our days are marked by the Earth's spin relative to it. Otherwise, we wouldn't have days and nights, and we wouldn't have weekends, which is scary to say the least.
You might have heard before that our sun is powered by nuclear fusion. All that gravity compresses the core, making it reach a ridiculously high temperature (tens of millions of degrees, give or take). Except, that's not hot enough for nuclear fusion!
All stars (our sun included) have to do nuclear fusion, which is what makes the shine. And all of them have nuclear fusion happening in their cores, which is what it means to even be a star.
Stars can be plotted on what we call Hertzsprung–Russell diagram (HR diagram). The group they fall into, tells us a lot about where they are in their life cycle. Our sun is in the "main sequence" group of stars, which means it's in the main part of its life. In other words, its main source of energy is Hydrogen Fusion. [1]
Hydrogen fusion is when 4 hydrogen nuclei (protons) come together to form one Helium nucleus.
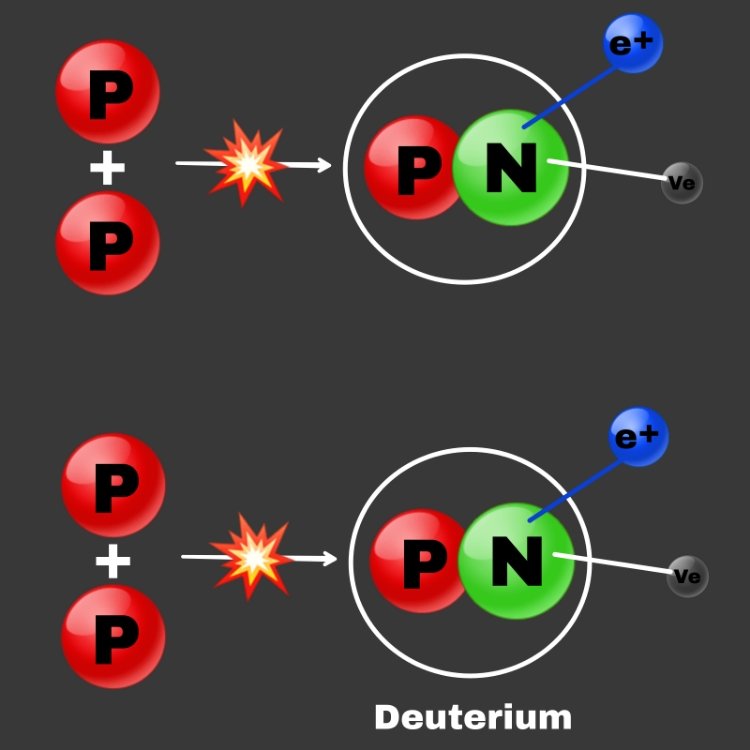
When the positron that is emitted from the beta decay encounters its antiparticle (An electron), the pair annihilate to form Gamma ray, which is the most energetic form of photons.
These Gamma rays eventually work their way up from the core of the sun, and out into space in the form of sunlight (even though that's a minor source). 🌞
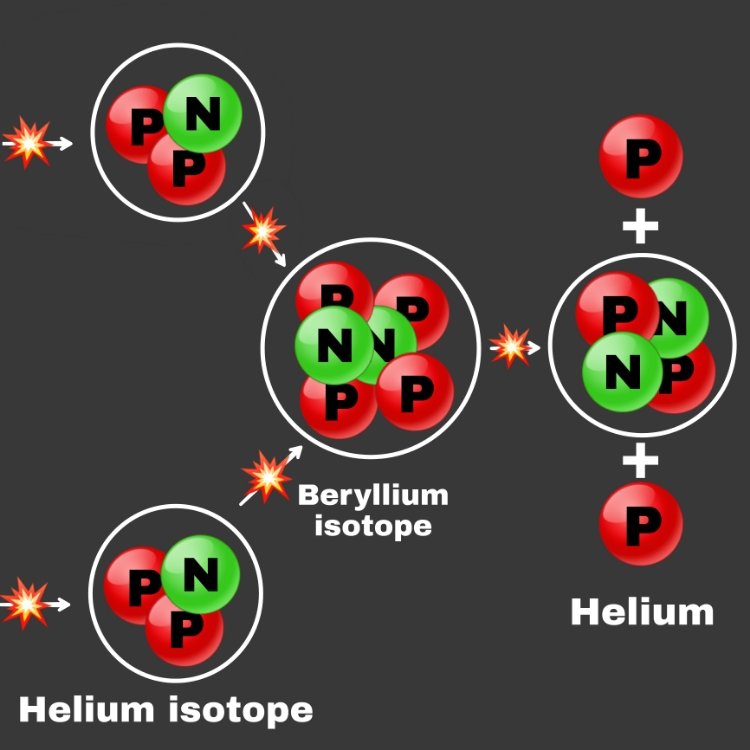
And energies are released each step of those processes. Starting with 4 Hydrogen nuclei (4 protons) and ending up with Helium. If we look at the atomic weight of those 4 Hydrogen atoms vs the weight of one Helium, we'd find the 4 Hydrogen atoms weigh slightly more than the resulting Helium atom. Mass gets converted into energy in that fusion process. And that tiny difference is responsible for nearly all the energy that you see coming from the sun. 🌞
Each fusion reaction releases about twenty-seven mega electron volts (27MeV) of gamma ray photons. Doing the calculation by E = mc2 that would be the equivalent to the mass of about 50 electrons. For comparison, our body would use 4 million million MeV's just to perform a simple task like going one step up a ladder. So it's a super tiny amount of energy as far as we are concerned. The sun however, produces a lot of energy because there's an enormous amount of those reactions happening at the same time. About 1038 reactions (that number is 1 followed by 38 zeroes) every single second. [3]

Fusion is inherently a quantum mechanical process. As mentioned earlier, 4 protons have to stick together for fusion to happen which isn't easy at all, the problem is protons all have positive charges, and as you know, similar charges repel each other. So the protons would repel each other if they get too close. At those tiny nuclear distances, there would be about 50 pounds of force pushing them apart. It's a ridiculous amount of force for subatomic particles. [5]
Get them just close enough, however, and the strong nuclear force would overcome that repulsion and can hold the protons together. And that's basically where those millions of degrees come from, the hotter the core is, the faster the particles would move and the more they would bump into each other.
The issue is, having protons bump into each other is not enough. Even that degree of 15 million Celsius is not hot enough to keep that fusion process going!

Quantum tunneling
When we try our hands with nuclear fusion here on Earth, we need hundreds of millions of degrees for it to happen, so those tens of millions of degrees in star cores aren't sufficient on their own to explain why the fusion is happening.
The real reason why nuclear fusion happens, is what we call Quantum Tunneling.
To overcome a barrier, you would need to go over or around it usually. Imagine a rolling ball that is trying to get over a slope, if the ball doesn't have enough energy to reach the top of that slope and go over it, it would just roll back down the direction it came from. But if it has enough energy to overcome the slope barrier, then it would roll up all the way and over that slope to the other side.
In the weird world of subatomic particles, things behave differently in non-intuitive ways compared to what we are familiar with in our normal scales. Probabilities are the best we could hope for to know the position of a particle for example. Each particle (including the protons that we are trying to get fused) are really like a tiny wave of quantum probability. Their behavior, where they are, what they are doing, it's all probabilistic.
And at that subatomic scale, there's a non-zero chance to even find a particle at the other side of that slope barrier if it's say thin enough, without it having the required energy to overcome the slope. It is as if that particle has tunneled through the barrier instead of going over it.
Hence the name "quantum tunneling".

Without that behavior of the quantum world (quantum tunneling), the stars, and our sun included, wouldn't be able to fuse anything, it simply wouldn't shine!
And it's a numbers game, the probability for the tunneling to happen is extremely small. As I mentioned earlier, to make fusion here on Earth in a reactor, we would need hundreds of millions of degrees even with the tunneling going on vs 15million degrees Celsius for the sun. Simply because we don't have as many atoms to play with, BUT the sun does.
That makes about 1057 protons, the core of the sun where fusion happens, however, has only about 12% of the sun's protons. So that's about 1056 protons in the core (Still a lot).
The chances of two protons would quantum tunnel together is only about 1 in 1028 chance. super terrible odds! But the number of the protons in the core makes up for that, as with THAT many protons bumping into each other, the chances of some of them quantum tunneling isn't that rare at all.
We only need 1038 such reactions to happen each second, and there's enough protons in the sun's core for it to happen for thousands of millions of years!
The sun is in a constant battle between two forces. Gravity which pushes on itself inwards, trying to crush it. And the nuclear energy that is getting released pushing against that gravity outwards, trying to tear it apart.
After about 5 more billion years, however, sadly our beloved sun would end up losing that battle once it runs out of its fuel. [3]
But until then, we can enjoy it while it lasts!

● Thank you for reading ●
● •
1- Stellar Neucleusynthesis | 2- Proton-Proton Chain reaction | 3- The Sun | 4- Quantum Tunneling | 5- Nuclear Fusion |

© 2022 @yaziris.
Congratulations @yaziris! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 70 posts.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out the last post from @hivebuzz:
Yaay!
Anyways, thank you guys!
If I don't even give up already..
You're welcome @yaziris, you're on a good way 😊👍 Have a nice day!
So, this whole post is just how we cannot create a fusion reactor. 😂
Quantum tunneling is so cool. Thanks for explaining it.
So you are saying if I start bumping to my wal again and again maybe one day I can get through. 🤪
Shhh, you weren't supposed to say that. 😅
Yeah, in my opinion, "as it has been for ages", we're like 30 years away from making one. 😋
About the wall thing, yeah.. Have to go FULL FORCE. Good luck ✌💥 😁
30 years doesn't look so far. Let's see if we can do it.
My walls are too thick for that... Maybe I should start with a much thinner door or it doesn't matter. 😁
Yup, except they said that 30 years before, and then 30 years before that too and so on. 😁
The thicker wall the merrier, teach those subatomic particles a lesson. 💀
I thought it was 50 years and not 30 ;)
😆
Interesting read.
What have you got to say about the supposed china's artificial sun.
Hi @cyprianj,
In regards to any nuclear fusion reactor. What is the purpose of making one? If it's to break world records for continuous operation, then sure, they have done that. (1,056 seconds at high plasma temperature)
But generally speaking, the in-flow of energy surpases any useful out-flow of energy by far. You'd never hear them (or any fusion reactor labs for that matter) speaking about how much useful energy they actually manage to get out of such reactor.
Nevertheless, it's still a step on the road of hopefully, one day, humanity can make an actually useful fusion reactor.
Thank you for your comment!
...!discovery 25...
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Delegate to us for passive income. Check our 80% fee-back Program
Your content has been voted as a part of Encouragement program. Keep up the good work!
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for new Proposal
Delegate HP and earn more
Interesting ✌️
Thank you!
Proud to know you @yaziris. I will try to read this on my iPad as I go to sleep.
That means a lot to me! 😊
The pleasure is all mine @agmoore. You're one of the most wonderful people that I've had the pleasure to know. ❤
Thank you for stopping by and for that lovely comment. Made my day!
Thanks again for this very clear blog on this topic. I have a small set comments, mostly with respect to one point.
First of all, I find the wording a bit confusing when you mentioned the following:
Technically, a proton cannot ”decay” into a neutron as proton is lighter. For this to happen, the proton needs to have some non-vanishing kinetic energy. In fact, we have instead the reaction proton + proton to deuterium + positron + neutrino (which happens super rarely relative to proton + proton to proton + proton). We should rather call this a beta process in my opinion than a beta decay (as done in some of my recent works on neutrinoless double beta processes here and there).
I have the impression that this is what you meant, but I found it a bit confusing in the way it is currently written.
Moreover:
and:
This is incorrect: there is no such a reaction in which two deuterium nuclei directly fuse into a helium-4 nuclei in the Sun.
In fact, one possibility to form helium-4 requires 6 hydrogen nuclei in the Sun. It proceeds through the formation of two Helium-3 nuclei that then fuse into one helium-4 nucleus and two protons (as mentioned in fact in your blog). Here, there is no need for a beryllium intermediate state. The two helium-3 nuclei can indeed fuse directly.
If we want to go through a beryllium-7 state, this is also possible. However, we need to rely on the fusion of a helium-3 nucleus with a pre-existing helium-4 nucleus, that then produces a beryllium-7 nucleus which decays into a lithium-7 nucleus, that then fuses with an extra proton to give rise to beryllium-8 which finally decays into two helium-4 nuclei. Here we go, but with many more than 4 protons in total!
The two chains happen regularly in the Sun, although the second one is a higher energy version than the first one.
Finally, when you wrote:
In fact, as you can see hydrogen fusion clearly contributes to the whole process (when deuterium and proton fuse to helium-3), but it is not alone. We have many other fusion processes all over the place. In terms in energy, most of it comes in fact from these other processes.
Cheers and have a good week!
Agreed. I should have stopped at "a proton gets transmuted" without the mention of a "beta decay". (Didn't have the term you mentioned -beta process- in mind).
I'm not used to being super accurate with the wording (which is something I've been learning to do since my very first article in stemsocial and my interactions with you hahaha) which I very much appreciate by the way :)
As for the rest of what you mentioned, I will talk in general as I completely agree and am aware of most of it.
The issue I'm always facing when writing is to what audience is it directed at? This has always affected my writing about physics subjects.
Many friends who are curious and like to read a bit about physics, have suggested to not go technical ("dumb your articles down" they say), in discord and in comments on older posts. And sadly, I have lost some readers because they felt I was going too technical even with my "child's play" way of describing things.
(I'm sure you can relate somewhat for the difference of writing/talking about a subject between your actual research and work -your physics papers, which are very hard even for me- vs -blogs on on here, which I somewhat can understand-)
For example, they are not interested in the detailed processes that take place but in the general idea. (Not that I even know all the details either, nor can they be included in the general purpose subject)
Almost everywhere in the "catchy" blogs and even in schools, they just say 4 hydrogen atoms combine to make a helium in fusion reaction, and that's that...
I wanted to go over a bit of the reactions, but mainly was about the general quantum tunneling idea being the key for fusion.
Lastly, I truly appreciate your input and corrections. And I want to ask you, what should I do? Be more technical and precise in my future articles (which I'm not sure I'd do great either xD ) keep them the way they are, or make them more catchy for the other audience (which means even less details and technicalities)?
In any case, I love your corrections and pointing out stuff. I learn a lot from them, correct and expand my personal knowledge. And I'm sure that applies to anyone reading the article/comments.
So for that, I thank you! And please keep doing it :)
Cheers! <3
The problem you mention here… is definitely a problem I face every time I write a blog. Unfortunately, I don’t know what to answer and I don’t even know whether there is any good answer at all (there is probably none).
I personally like to avoid enforcing too many simplifications, as this is how important facts are often put under the carpet. Doing so could hit us very strongly much later (the original papers on the 2021 Nobel prize in physics on climate change are good examples on why we should not always over-simplify as much as we could).
However, it is important to simplify a bit, as you said, in order not to lose our audience. What I found out is that no matter how much I simplify, there are always people complaining that this is too technical and people happy with the content. This is the most puzzling part :)
Moreover, most of these problems are non existent when we discuss with people live, in front of a good drink for instance (science cafes are cool!). Of course, the situation changes in the context of a blog. Here, there is simply no way to tune the speech depending on the audience understanding and how people get lost or not.
Because of the above I decided to keep the following structure in my blogs: simpler first and last sections that are sufficient to get the main ingredients of the blog (and learn some physics in the process). Then the middle sections are more technical. This way of writing makes me happy, and does not seem to have affected the readership. At least I have never got any negative feedback (it is maybe about time :D ).
What I mean here is that you probably need to follow a path that makes you happy. The rest… well it is less important. Personally, I find all your posts very fine. They allow to discuss some cool physics, and with this respect their purpose is filled. Clarifying things like I do once in a while is not a big issue at the end of the day. The comment section is there for this purpose, and can be read by anyone who would like to know more.
In short, I do not see why you should change anything. But this is only my own opinion, which is worth what it worths...
Your opinion is worth a lot, you went through many great points and everything you said was spot on in my opinion.
I will keep doing what I'm doing I guess, "try to keep it as simple as possible, but not simpler". (Although that can be subjective too xD )
But yeah, that climate change example was a great reminder.
Yes, exactly. This is why I was emphasizing the fact that I appreciate your comments! Didn't want you to think otherwise.
Noted! See you on your next blog post :DTo conclude:
This is definitely the way to go in my opinion ^^
PS: I am still waiting for the negative feedback ;)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
I would like to join this community would you please tell me any navigation for join and how to post on stemgeeks?
Hi @alfazmalek02, this is StemSocial community, you can find an invite to their discord channel in their comment on this article.
As for stemgeeks, you can go to stemgeeks.net and use it for posting to that community or to this one.