Children Of The Stars 🌟 Where Do The Elements Come From?

Everything we see around us is a complex composition of trillions upon trillions of atoms. Each kind of atom has different properties, largely determined by the number of protons it contains within its nucleus.
Shortly after the big bang, the universe only consisted of the lightest elements. Hydrogen, Helium, and Lithium, along with some of their isotopes.
In nature, however, we can find a range of much heavier elements, as heavy as Californium the 98th element on the periodic table.
If no atoms heavier than Lithium were created at the beginning, then where did all those other heavier elements come from?
Most importantly, Carbon, Nitrogen, and Oxygen. Without those, life wouldn't have been enabled to exist, and there wouldn't be us asking and reading about such questions.
The origin of elements in the periodic table is an active area of research. And while we are still learning the details, we can discuss some information based on the best theories we currently have.
Origin Of The Elements

There are 118 known elements that we have discovered so far, the heaviest 20 (which I painted over in red) are all man-made in labs, they do not exist in nature by themselves.[1]
Which leaves us with 98 elements to wonder about their origins.
During the first minutes of the Big bang, a process called nucleosynthesis took place, this is where the newly formed protons and neutrons started to form nuclei. The cores to what later would become the first atoms.[2]
At that point, there were no neutral atoms, only the charged nucleus of atoms, mainly just protons, which are the nuclei of Hydrogen atoms. Positively charged without any electrons orbiting them.
At that stage, only the 2 lightest nuclei formed, about 75% Hydrogen (a single proton), and about 24% Helium (2 protons and 2 neutrons) along with some trace amounts of their isotopes and Lithium.[2]
The nucleosynthesis ended when the universe was only about 20 minutes old. And while we only had those 2 forms of light nuclei at that point, all that we have today was formed later on from those.
[3]
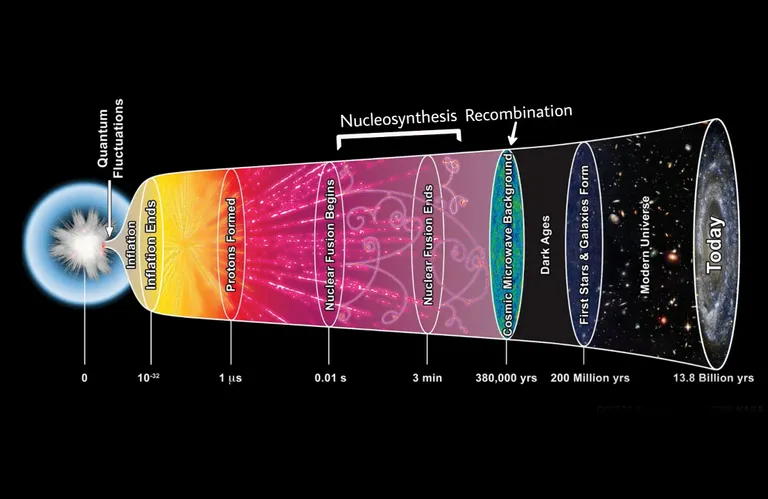
Fast forward about 380,000 years. The universe cooled down substantially! From about 5 billion Kelvin to about 3,000 Kelvin.
A process called Recombination took place. That was the point where the universe was cool enough so the nuclei could capture electrons and become neutrally charged atoms instead of just free-floating charged nuclei and electrons in a plasma state. Prior to that, energies and temperature were too high for them to stick around and be bound in orbitals around the nuclei.[4]
2 down, 96 to go!
After the Recombination period, the universe went dark. During those "dark ages" however, Hydrogen gas condensed into stars, soon enough, the universe came out of that dark period, as the first stars formed and began to shine. 🌟
Stars shine, thanks to a process called Fusion. This takes place in the core of the star, where pressure and temperatures are high enough to initiate the fusion. In those early stars, for example, 4 Hydrogen atoms combine together to form a Helium atom.[5]
I won't get into much details on how that process works as it's beyond the subject of this article, but this is basically how some of the heavier elements form.
Something like our sun, however, which is considered a medium-sized star compared to other stars, can only carry that fusion process to produce some heavier elements, up until elements like Carbon or Nitrogen.
As the element gets heavier, the energy you get from fusing it becomes less and less.
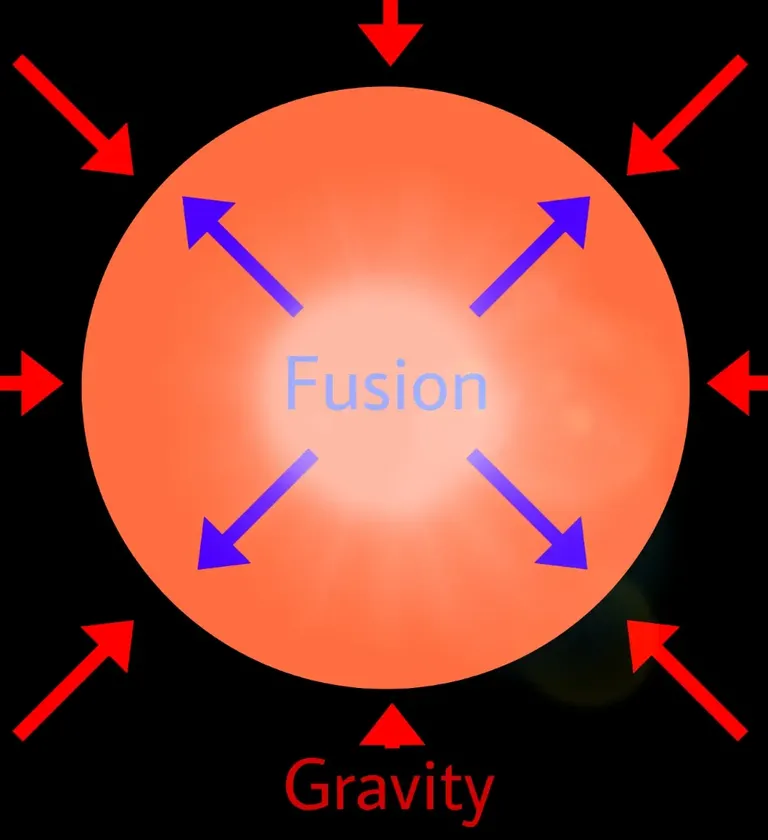
Smaller stars cannot produce enough radiation pressure when fusing heavier elements, thus losing the battle against gravity, eventually collapsing to form small White Dwarfs.
Fusion stops, the star cools, and it stops shining!
Bigger stars though can win that battle against gravity up to point.
As those larger stars begin to die, they start to produce elements in their layers, heavier elements closer to the center and lighter elements as you go to the outer layers closer to the surface. At some point, even in bigger stars, the fusion process would require more energy than the one produced from fusion.
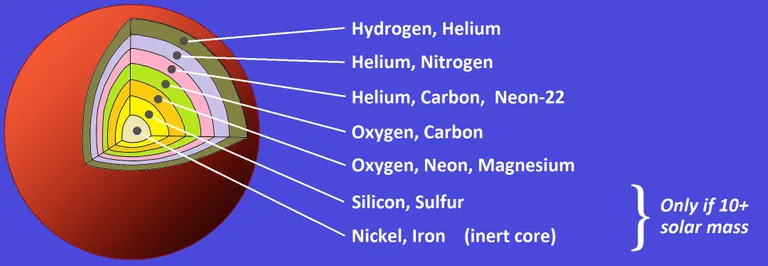
Large stars can produce heavier elements up to Iron. But the fusion process at the core stops at Iron.[5]
Unlike the elements before it, Iron releases no energy at all when fused, as it has the highest nuclear binding energy of all other elements, it has the most stable nucleus.[6]
The fusion process stops at Iron, but it's remarkable how those large stars can fuse all those elements up to iron simply from Hydrogen atoms.
Halfway there!
Well, that fusion process in stars explains the origin of about half of the elements in the periodic table.
To get the other half, however, those massive stars need to die! 💥
When such massive stars die, they explode. This can be seen even from the earth and is known as a Supernovae. As a dim star can briefly shine with a luminosity of an entire galaxy. Lasting very briefly, usually no more than a couple of minutes.
The star collapses under its own weight when it doesn't have enough energy anymore to counter the gravity. A million times the size of the earth, suddenly collapsing to a size of 30 Kilometers in only about 15 seconds. It causes enormous shockwaves, exploding the outer part of the star. Leaving behind a small dense core, usually a neutron star or a blackhole.[7]

Elements beyond Iron are not produced in the core of stars, rather, they get formed in those explosions!
The extreme temperatures in a supernova can reach billions of degrees, which drives additional nucleosynthesis for elements heavier than Iron.
These explosions yield heavier elements all the way from Iron up to Zirconium. (40th on the table)[8]
Where do the rest come from?
Smaller stars are made from the remnants of previously exploded stars (supernovas).
From nebulas of gasses from many exploded stars in the past.
So even though a small star like our sun cannot produce elements much heavier than Nitrogen, it contains heavier elements even up to iron.
Furthermore, lower mass stars produce excess neutrons as a byproduct of fusion. And as they're neutrally charged, it's relatively energy-efficient for a free neutron to get captured by the nucleus of a heavier element.
When the free neutrons merge with these heavier elements they produce an even heavier isotope of the element. Over time, due to the weak force, some of those neutrons transmute, turning into protons inside the nucleus.[9] This process can continue, to produce heavier and heavier elements. And thanks to that, stars can make heavier elements from strantium all the way to Lead and Bismuth. (83rd on the periodic table)
Similarly, When 2 neutron stars merge, they can free up an abundance of neutrons. Those neutrons can be absorbed by heavy atoms near those neutron stars. Those heavy elements become even heavier over time as some of the neutrons transmute into protons.[9] All the way up to Plutonium (94th).
The last 4 heavier elements, all have very unstable isotopes, and they can only occur naturally near radioactive sources. But they are extremely rare.

98% of all visible matter in the universe is made only from 2 of all the elements, Hydrogen, and Helium!
All the remaining 96 naturally occurring elements make up only 2% of everything that we can see. All the atoms in our bodies are part of a very minuscule portion of the visible matter in the universe.[10]

As the stars died they gave us life. We are indeed the children of the stars! 🌟

● Thank you for reading ●
● •
1- Synthetic Elements | 2- Nucleosynthesis | 3- Chronology of the universe | 4- Recombination (cosmology) | 5- Nuclear Fusion | 6- Iron peak | 7- Supernova | 8- Supernova nucleosynthesis | 9- Nuclear transmutation | 10- Abundance of the chemical elements |


Banner by #theterminal
Oh wow. This means the atoms in our bodies are composition of hydrogen and helium. This is interesting and detailed research work. Great. 😃
Hydrogen and helium were the first to form in the begining of the universe, the rest came after that. 😁
Our bodies contain many different heavier elements which had formed later on.
Thank you for stopping by.
!PIZZA
PIZZA Holders sent $PIZZA tips in this post's comments:
@yaziris(1/5) tipped @ladytitan (x1)
Join us in Discord!
Hydrogen and Helium sound familiar, I think from a nursery rhyme.
I've never really read much about the scientific explanation to how we all got here as its kinda complicated for my simple mind😅
CHILDREN OF THE STARS _ LOVE THAT AS IT'S MY NAME🌟❤️
Well atleast you made it quiet easy.
Well researched topic, which I'm not surprised because that's you, your very thoughtful with your post.
Much love to you💗
I actually thought you'd say that about the stars if you saw this post when I was writing it. 🤣
Thank you for your sweet words, much !LUV to you too! 🌟💛🌟
@yaziris(1/1) gave you LUV. H-E tools | connect | <><
H-E tools | connect | <><
😂That's what caught my eye and insaid
"Let me READ and find out you're upto"
😂
You wrote beautifully, so proud🤗
Congratulations @yaziris! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 800 comments.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out the last post from @hivebuzz:
This was cool to read. This reminds me the fact that we are all made of star-stuff, to rephrase Carl Sagan :)
By the way, did you know that it is possible that heavier elements are actually dominantly formed by the merging of neutron stars. This is an option that emerged from recent gravitational-wave astrophysical observations.
Cheers!
Thank you so much for reading and for your comment, I'm really glad that you liked it :)
I mentioned this I think very briefly here
I just didn't know that this is actually the dominant way of heavy element formation, thank you for that info!!
Much appreciated, cheers! :))
The importance of neutron star mergers was debated like a year ago, and I must admit that didn't see any update on the outcome of the debate. I quickly checked online, and it turns out that this is still under debate (at least this is what this recent article from last year tells us).
Therefore, we should say that heavier elements could be dominantly produced in binary neutron star mergers. The conditional tense is important ... for now ^^
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.