ORGANIC CHEMISTRY: ALKENE & ALKYNE FAMILY

Hello hivers, hope things are falling into places there? I welcome you to enjoy another interesting post on my blog. I discussed fundamentals of organic chemistry in my last post where I talked about alkane, its properties and naming.
Taking about alkanes only without touching alkenes and alkyne will be unjust. I will start with alkene.
ALKENE
Alkenes are unsaturated hydrocarbons having double bonds in the system. They are also called 'olefines' which means oil-making, given to them because of the ability of ethylene(C2H4) the first member of the series to produce liquid oil substance upon contact with chlorine. The oily substance is known to be ethylene chloride, C2H4Cl2. Alkenes has the general formula CnH2n.
For instance, if the number of carbon present in the alkene compound is 4. Its molecular formula will be C4H(2×4) which makes it C4H8(butene).
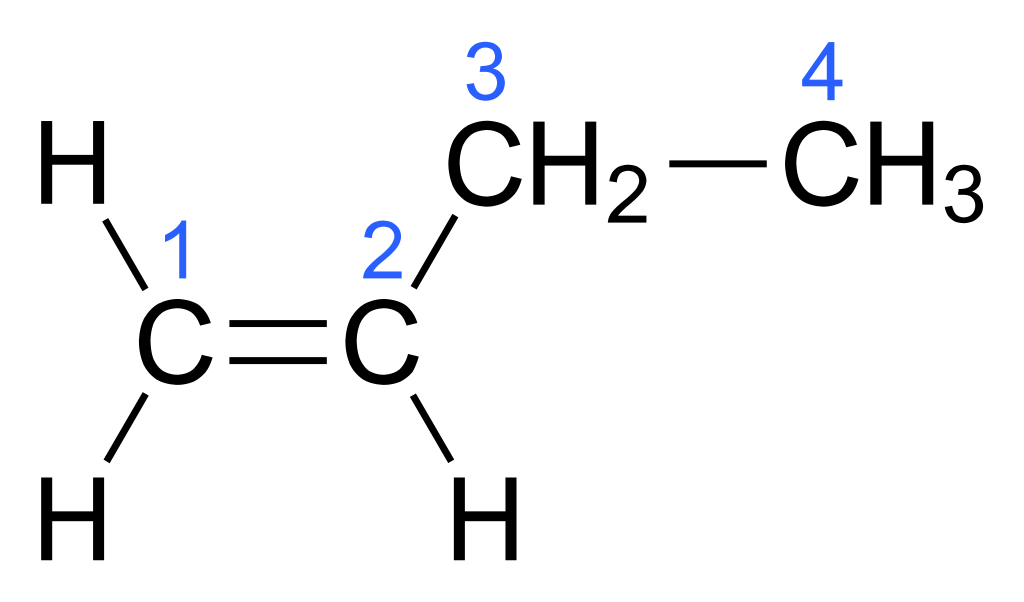
STRUCTURE OF ALKENES
The distinguishing feature of the alkene is the double bond that exists between two adjacent carbon atoms. The carbon atoms linked by double bonds are in the state of sp2 hybridization. Each of them forms three sigma bonds which lie on the same plane at the angle of 120°. The second type of bond, a pi bond, is made by lateral overlap of unhybridized orbital of adjacent carbon atoms. The electron clouds of the pi bond envelopes the sigma bond, lying above and below the plane on which the atoms lie. These two types of bonds sigma and pi are different in nature, in strength and geometry.
The side overlap of the unhybridized orbital to form the pi bond makes the double bond of alkene much shorter than the single sigma bond.
NOMENCLATURE
According to modern international rules of IUPAC, the names of alkenes are constructed in the same manner as alkanes, the names end in -ene. The position of the double bonds is indicated by a number which corresponds with the carbon from which the double bonds begin. The longest chain that is located must necessarily include the double bonds, the numbering of the carbon atoms also gives the lowest possible number to the double bonds after which the carbon substituents are numbered. The numbering will start from the position of the double bond nearer to the first carbon.
-3-methylpent-2-ene_200.svg.png)
In the structure above, the numbering starts from the last carbon and not the first simply because the double bond is nearer to the last carbon than the first.
ALKYNES
Alkynes are unsaturated hydrocarbons just like the alkenes; having tripple bonds in the system. They are also called 'acetylenes' which is the first member of that group, ethyne(C2H2). Alkynes has the general formula CnH2n-2.
For instance, if the number of carbon present in the alkyne compound is 5. Its molecular formula will be C5H(2×5-2) which makes it C5H8(pentyne).
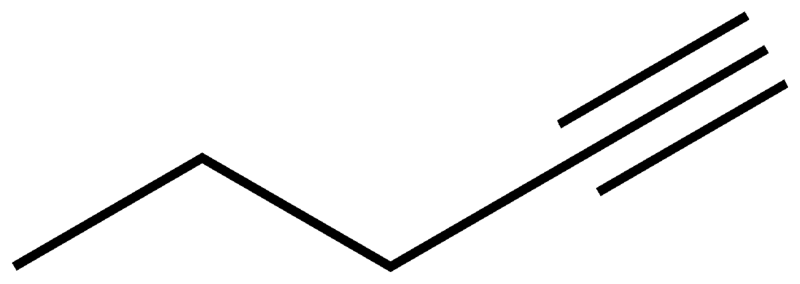
NAMING IN ALKYNE
Naming in alkyne is the same as in aliens except that there is three bonds in alkyne and should have the suffix -yne.
Note that the position of the triple bond must be indicated when naming the compound.
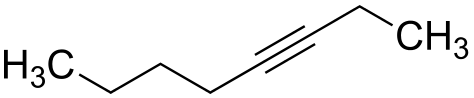
The location of the triple bond is indicated above and it is attached to carbon-3.
In conclusion, alkenes and alkynes have more similarities that alkane only that one has double bond while the other has triple bond. Their general formula differs by 2. An example of alkene is kerosene which can be used for cooking.
REFERENCES
Alkyne
Introduction to organic chemistry

Organic chemistry is everything ❤️ Good job, thanks for sharing with us.
!discovery 20
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Good one,this may help a fellow chemistry student. keep it up :)
Thanks for the cool comment. We are there to help each other.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Congratulations @sirpee6! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Do not miss the last post from @hivebuzz:
My knowledge on chemistry are very poor, thank your posts 😊