ALCOHOLS IN CHEMISTRY
Alcohols are products of the replacement of hydrogen of hydrocarbons with hydroxyl group (-OH). The general formula of a simple alcohol is R - OH, where R is a hydrocarbon radical. Aliphatic alcohols are divided into saturated and unsaturated, depending upon the nature of the hydrocarbon radical.
When the hydroxyl group is a derivative on the side chain of aromatic hydrocarbon, the compound is called aromatic alcohol and when the hydroxyl group is attached directly to a benzene ring it is called phenol. The phenols will be considered later, since they form a distinct group of their own. There are also primary, secondary and tertiary alcohols depending on the type of carbon bearing the hydroxyl group. Most common are alcohols with one hydroxyl group called monohydric alcohols, while two hydroxyl in a molecule makes a dihydric alcohol. A molecule with three hydroxyl groups is a trihydric alcohol. There also exist polyhydric compounds.
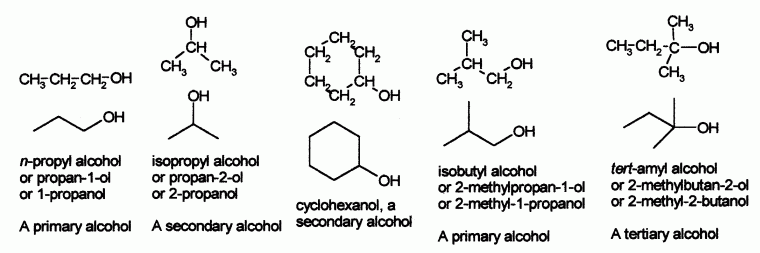
Nomenclature
The principal chain is the longest carbon chain that contains the hydroxyl group. A numeral indicates the locant carbon of the hydroxyl group but this number is not necessary when the hydroxyl group is on carbon 1. Numerals are used to indicate the locants of all other substituents all of which are at the prefix. The numbering is such that the hydroxyl carbon is given the lowest possible number. The principal chain bears the suffix -ol*.
Methods of Preparation
- Reduction of aldehydes and ketones
Catalytic reduction of cabonyl compounds to secondary and primary alcohols is widely used. Aldehydes produce primary alcohols whole ketones give secondary alcohols. The most promising of the catalysts is the active Raney nickel which permits hydrogenation in glass apparatus at low temperatures and pressures. Metallic hydrides are excellent reducing agents for cabonyl compounds the hydrides include, lithium aluminum hydrides and also sodium borohydride which may be used in aqueous or methalonic solutions, does not reduce esters, acids or nitriles and so may be used for selective reductions.
A convenient procedure for the reduction of ketone involves the periodic addition of small pieces of sodium to a slowly stirred ethereal or benzene solution of the ketone. - Meerwein-Pondorf-Verley Reduction
The cabonyl compound is heated with aluminium isopropoxide in isopropanol. The alcohol is oxidized to acetone while the cabonyl is reduced to alcohol. The reaction is in equilibrium, the reduction of the cabonyl is completely accomplished by steady removal of the acetone produced during the reaction by distillation. This method was reviewed by highlighting the experimenting conditions and limitations, aluminum isopropoxide is superior to all other alkoxides and it gives better yields and the technique for determining the end of the reaction is simple. This reduction reaction is specific for aldehydes and ketones. It is most useful for the preparation of Olefins, halo and nitro alcohols from the corresponding aldehydes and ketones. - Oxidation-Reduction reaction
This reaction is specific with aldehydes with no alpha hydrogen. It is an intermolecular oxidation-reduction reaction, popularly called cannizzaro reaction. - Reduction of carboxylic acids and esters: Lithium aluminium hydride (LiAlH2) has served as a powerful reducing agent that will reduce carboxylic acid, acid chlorides, esters and anhydrides to primary alcohols. Taxtones are converted to diols. The reduction of esters by sodium in alcohol, bouveault-Blanc procedure is widely used.
- Hydrolysis of Alkyl Halides: A general methid of preparing alcohols is the hydrolysis of alkyl halides with aqueous alkali or silver oxide suspended in water. Since the same conditions necessary for substitution reaction in a tertiary alkyl halide are the same conditions for elimination, the simple method of hydrolysis produces a large amount of alkene. The conversion of dihalides to diols through the diacetate derivative is in some cases more convenient than the direct hydrolysis. The diesters are prepared by heating dihalides with sodium or potassium acetate in acetic acid or ethanol.
- Hydrolysis of esters
By heating esters with dilute sulphuric acid under pressure alcohol are produced. Diethyl ester is hydrolyzed to form ethanol. This is a very important process in the industry. Esters that are formed as by-produced during the preparation of alcohols are converted back to alcohols in this way. - Reactions of Grignard reagent with aldehydes and ketones
The grignard reagent provides the routes to obtaining primary, secondary and tertiary alcohols. Formaldehyde yields a primary alcohol while any other aldehyde gives secondary alcohol. Ketones react to give tertiary alcohols. - Fermentation of Carbohydrates
The age-long method of preparing alcohols by fermentation is still in use today. The fermentation of carbohydrates give ethanol as major product but other alcohols such as propanol, primary butyl alcohol are obtained alongside the major product. Wastes of wood-working industries, such as sawdust chips, are hydrolyzed in 5% sulphuric acid under pressure to glucose. The hydrolyzate is neutralized with lime to precipitate CaSO4 filtered and fermented. - Hydration of alkenes
This is an industrial method of obtaining alcohols. The alkenes are available as product of cracking in the petroleum industries. Today, about 90% of the world ethanol is produced this way. Other olefins produce secondary and tertiary alcohol since addition of water molecule obeys Mackovnikov's rule.
Reference
Organic Chemistry- The fundamentals by Owolabi B.J. and Olarinoye N.O.
Enjoyable manuscript from you! This is well explicit information.
Cheers
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Congratulations @sirpee6! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz: