OCEAN ACIDIFICATION, A PROBLEM OR A SOLUTION TO CO2 EMISSIONS
(Edited)

Greetings dear and appreciated readers who make life in this prestigious platform, the topic that I will develop below is related to the side effects that has caused us the large-scale production of Carbon Dioxide (CO2). One of the main consequences of this chemical is the acidification of the oceans.
INTRODUCTION
Environmental problems are a situation that has been affecting the planet earth for many years, as a consequence of the intervention of man's hand, who has caused an imbalance in the ecosystems and biodiversity of the different species that inhabit the earth. One of the main culprits is the emission of different gases into the atmosphere that have generated climatic changes such as the greenhouse effect, global warming and acidification of the oceans.
Global warming is the result of the increase in the temperature of planet Earth, as a consequence of the accumulation of different chemical gases generated in the daily life of human beings, which are directly responsible for the increase in sea level and the diverse climatic changes that are generated on the planet.
Since 2004, research has begun on a new natural phenomenon, which was called the solution of the emission of gases such as Carbon Dioxide (CO2), which is one of the main chemical substances that are generated in greater proportion on the planet, therefore when it is emitted into the atmospheric air is dissolved by the water of the oceans, causing the water to become acidic and thus creating an alteration in the marine ecosystem to such an extent that their growth and reproduction is being affected by this phenomenon.
The main objective of this publication is to provide some insight into the theoretical conceptualization of ocean acidification, how ocean chemistry intervenes in maritime development and to indicate some possible solutions to help mitigate this environmental problem that is gradually killing our species in the sea.
OCEAN ACIDIFICATION
It is a chemical process by which the pH of water decreases to an acidic point, as a result of the large amount of absorption of carbon dioxide (CO2).

The absorption of CO2 by water is of great help to the planet earth, since less of these gases reach the earth's crust and thus do not contribute to the greenhouse effect.
The acidification or acidosis of the oceans is a serious problem that is affecting marine species, as it alters their ecosystem and the food chain, this takes place because the CO2 found in the air dissolves in the water to give rise to Carbonic Acid (H2CO3), which affects the flora and fauna of the seas.
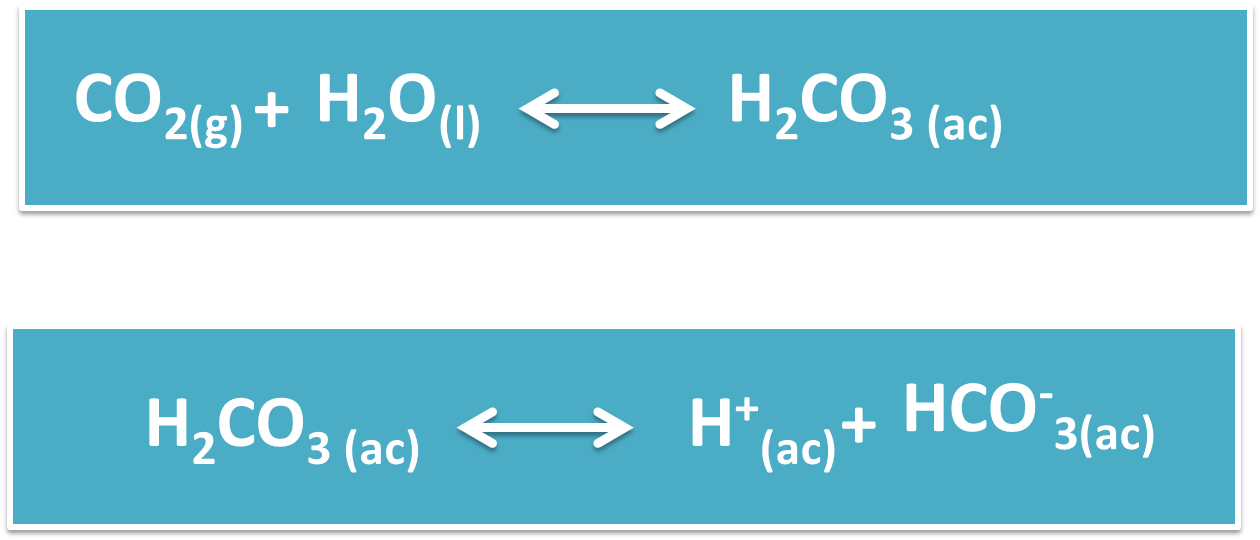
OCEAN ACIDIFICATION HELPS OR HURTS PLANET EARTH
We are in a disjunctive dilemma when we think if the absorption of CO2 in the ocean is a help for the conservation and preservation of the different species. However, it is an equally worrying situation, because this acidification has been increasing since the beginning of the industrial revolution, as a consequence of the large amount of CO2 that is released into the environment during the development of its processes.
.jpg)
Global warming and ocean acidification are two very similar phenomena, known in this field as twin brothers, since both threaten terrestrial life and are caused by CO2. However, it is important to note that global warming is produced by a set of greenhouse gases, which generate a progressive increase in the temperature of the earth's surface causing glaciers to melt, while acidification is caused only by the dissolution of CO2 found in the air into the water of the oceans.
ACIDOSIS AND ITS EFFECT ON THE CHEMICAL COMPOSITION OF MARINE SPECIES
Among the main side effects that have resulted from the decrease in the pH of the oceans is the formation of the shells of various marine species, such as calcareous plankton, sea turtles, clams, oysters, and sea urchins. These crustaceans are characterized because their crusts are formed by calcium carbonate (CaCO3), which are seriously affected by the acidification of the water, because it is given as a result of an increase of hydrogen ions and by increasing this directly decreases the concentration of carbon ions, which play a key role in the formation of carbonates.
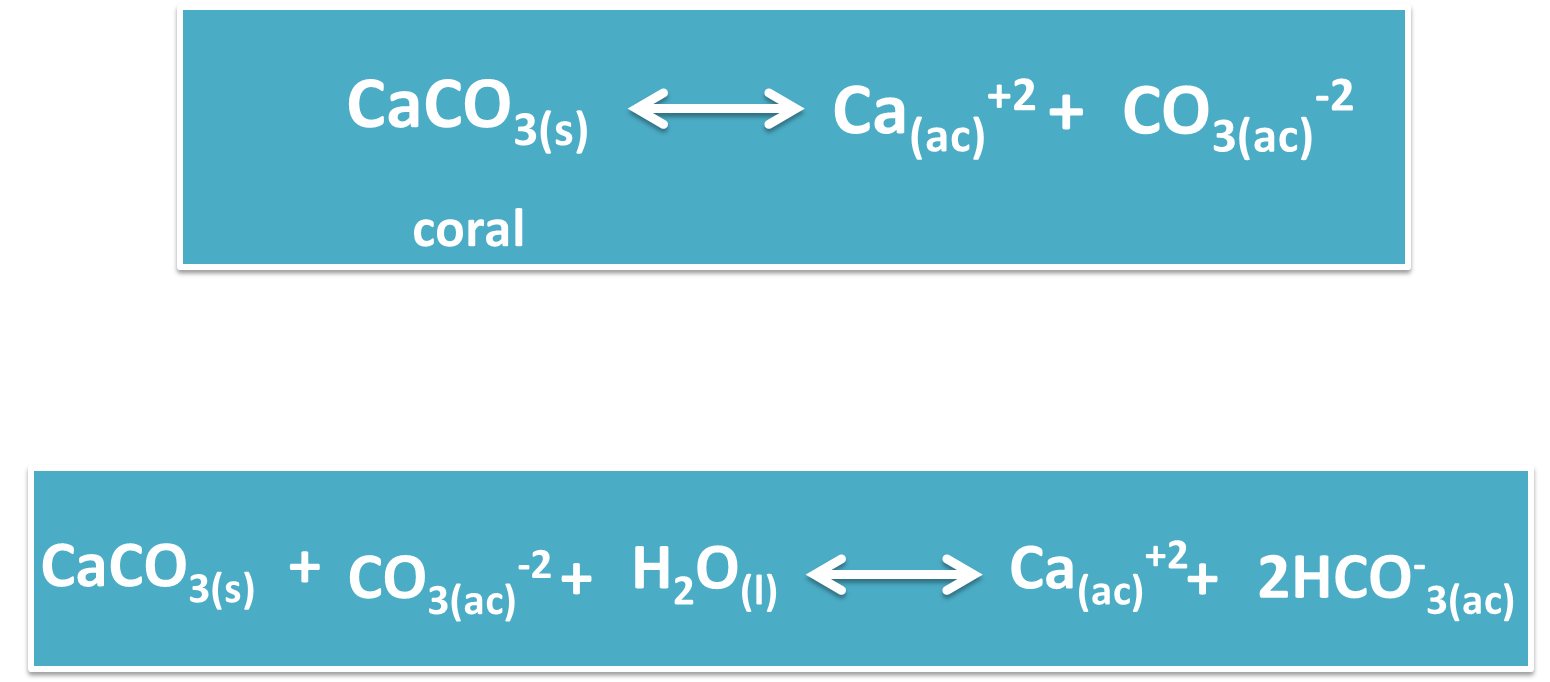
Similarly we have the case of corals, which need this chemical substance to reproduce asexually, so this is one of the species that are in danger of extinction because when one dies it cannot be replaced.

All of the above described makes these species more vulnerable to predators, since it is their shell that protects them from predators and as their formation is being affected by this fact, they have few options for survival and their population is declining.
In the same way, acidification affects clownfish, since this change in pH makes them disoriented and lost in the immensity of the sea, causing a progressive decrease in their species..
NOISE POLLUTION
With the prolonged increase in ocean acidification, the water has lost up to 40% of its capacity to dampen noise, which affects all marine species because it is creating a change in their ecosystem. This situation alters the sound waves emitted by marine animals, the orientation and feeding of the different aquatic species.
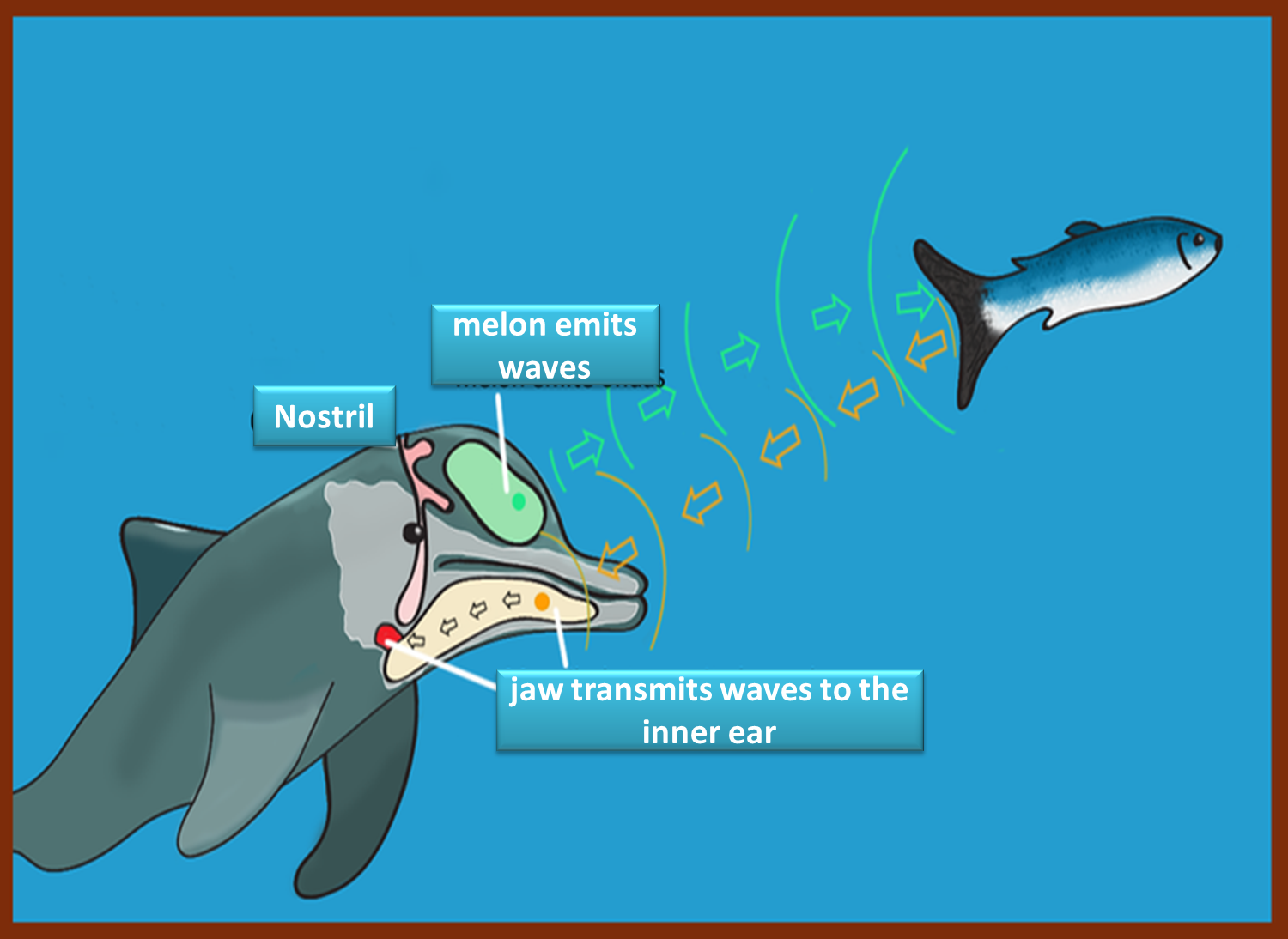
With this phenomenon described, the increase in sunlight entering the water and the variation in temperature is modified, which causes an imbalance in the habitat of marine species and if they do not get used to these changes, they may end up becoming extinct..
EXTINCTION OF MARINE REPRODUCTION
With the chemical variation that is taking place in the oceans, the growth of coral reefs is being affected, as well as the reproduction of the different marine species, since their development in such corrosive waters causes deformations that do not allow them to reach adulthood, making their survival more difficult and therefore affecting their food supply, biodiversity and fishing.

ALTERNATIVE OR SOLUTION
Is it possible to stop emitting carbon dioxide into the environment? No, so how can we contribute to the decrease of CO2? Even if we were to go back to the time before the industrial revolution, we would have to spend millions and millions of years for the water in the oceans to reach its normal pH..
The most viable option is to implement different environmental awareness programs with the sole objective of calling for reflection in relation to the large-scale emission of CO2, since if we stop using fossil fuels and use non-renewable products we can improve our quality of life, this would be in the field of electricity production, since it is in this process where most CO2 is generated into the atmosphere, so it is recommended the implementation of new alternatives in the energy area, such as wind, solar and hydrogen energy.

In the same vein, it is recommended that the use of automobiles, trains or any other means of transportation that generates CO2 emissions into the atmosphere be reduced.
FINAL CONSIDERATIONS
Even though ocean acidification is an environmental problem that seriously affects biodiversity, ecosystem and reproduction of marine species, it is not being considered as a priority by the competent bodies, without taking into account the negative effects that this brings not only to aquatic species but also to us as consumers of them.
One of the most latent consequences of this phenomenon is the possible disappearance of coral reefs, along with the weakening of the carcasses of marine species that require carbonate for the formation of their shells, which puts them at risk from predators.
The changes that have been generated with the acidification of the oceans, these species find it difficult to adapt and even more so those dependent on the formation of the skeleton and shells with carbonate. Examples of these are oysters, mussels, crabs and lobsters.
MATERIAL CONSULTED
Harrould Kolieb, Ellycia. Acidification: how does CO2 affect the oceans?. Source:
Laffoley, Dan. (2017). Introduction to ocean acidification: what it is, what we know and what can happen. Source:

0
0
0.000
Electronic-terrorism, voice to skull and neuro monitoring on Hive and Steem. You can ignore this, but your going to wish you didnt soon. This is happening whether you believe it or not. https://ecency.com/fyrstikken/@fairandbalanced/i-am-the-only-motherfucker-on-the-internet-pointing-to-a-direct-source-for-voice-to-skull-electronic-terrorism
Electronic-terrorism, voice to skull and neuro monitoring on Hive and Steem. You can ignore this, but your going to wish you didnt soon. This is happening whether you believe it or not. https://ecency.com/fyrstikken/@fairandbalanced/i-am-the-only-motherfucker-on-the-internet-pointing-to-a-direct-source-for-voice-to-skull-electronic-terrorism
Congratulations @sidalim88! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
Your next target is to reach 900 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out the last post from @hivebuzz:
https://twitter.com/ChavezMiladis/status/1441098196789325825
The rewards earned on this comment will go directly to the person sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Greetings @steemstem, thanks for supporting me in my work we will continue working on more scientific content.