How to promote anti-tumor sentiments in the immune cells. New lessons from an old friend - gut bacteria.
Imagine any regular guy living in your street. Now imagine that one day he becomes a mafia boss. He might even be loved by the cells around him but is detrimental to the city in the long run. This is your tumour cell. Soon, he makes a big cartel. This cartel is the tumour. Other than cancer cells, this cartel has all kinds of supporting cells. Even immune cells. The question that comes to the mind is wtf are the police or the immune cells doing l? Why are they not arresting or killing the gangsters? Even though so many of them are right there? Why are they colluding with the bad guys?
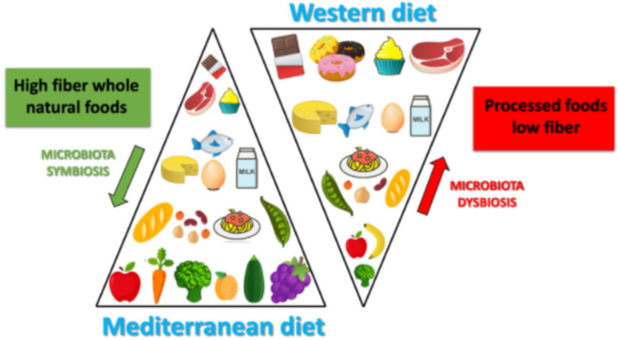
The kind of diet we eat affects the kind of bacteria that live in our gut. This affects a shit load of things in the body - including but not limited to cancer development and its prognosis. Image by Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A. | CC BY-SA 4.0
It has been often highlighted that our lifestyle and our diet influence our risk of getting cancer. It also affects the prognosis if we do get cancer. While the diet is not the magic bullet to fight cancer, it can definitely aid the therapy1, 2.
However, the question remains how?
While diet can affect our cellular metabolism directly, there is another indirect way.
The diet influences the kind of bacteria that live in our gut, and even on our skin 3, 4. These bacteria not only outnumber the number of human cells that are there in a human body, but they also influence a lot of shit that goes on in our body. This includes regulating the immune responses in the body. In a nutshell, even if you are not what you eat, they depend on what you eat, and they seem to have the power to change you based on what you eat.
Mind = Blown!
This week, I came across this paper in my email yesterday, and it totally blew my mind. I mean, sure. I knew that immune cells interact with gut bacteria. I also knew that gut microbes are correlated with the risk of cancer and outcomes of cancer therapy. I sure as hell knew the pathways which cancer cells exploit to influence immune cells in their favour. I even wrote a small blog on it[5]. But, I never really imagined that a third party sitting in the gut influences what the immune cells would do in response to a tumour. I mean, this is like you come up with a brilliant plan as a child to convenience your parents to buy you an Xbox. Instead, those nosy neighbours convenience your parents that how bad is the Xbox for you. Like, EXCUSE ME!
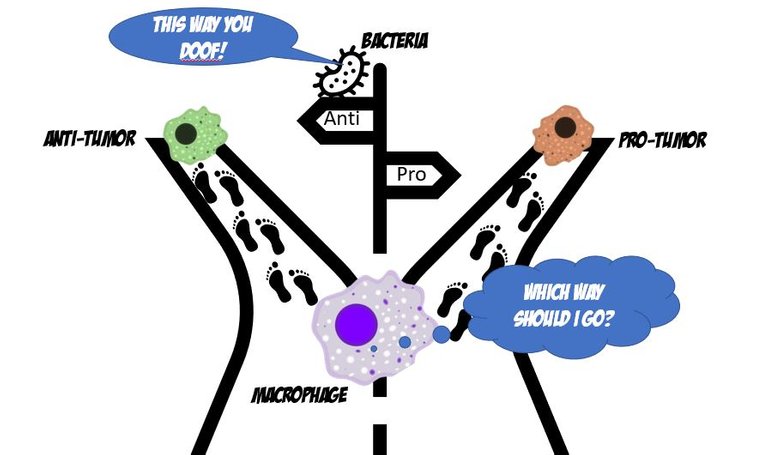
Is that really such a hard choice to make? Doofus immune cell! Listen to the damn bacteria there!Illustrated by scienceblocks using copyright free icons from MSoffice, and macrophage by A. Rad, Mikael Häggström, Spacebirdy, CC BY-SA 4.0.
This paper 6 I am talking about today studied the role of the gut microbes in the programming of immune cells to be either pro or anti-tumour. To do so, the authors first implanted tumours in regular mice that grew up with germs (barring some pathogenic germs). And, in mice that grew up in a completely sterile environment since birth and were hence completely germ-free 7. They analyzed gene expression signatures of single cells in the tumour to identify their type and what they might be doing there. Turns out, in germ-free mice the gene expression signatures from macrophages (a type of immune cell) matched that of pro-tumour ones.
What you are supposed to do v/s what do you do?
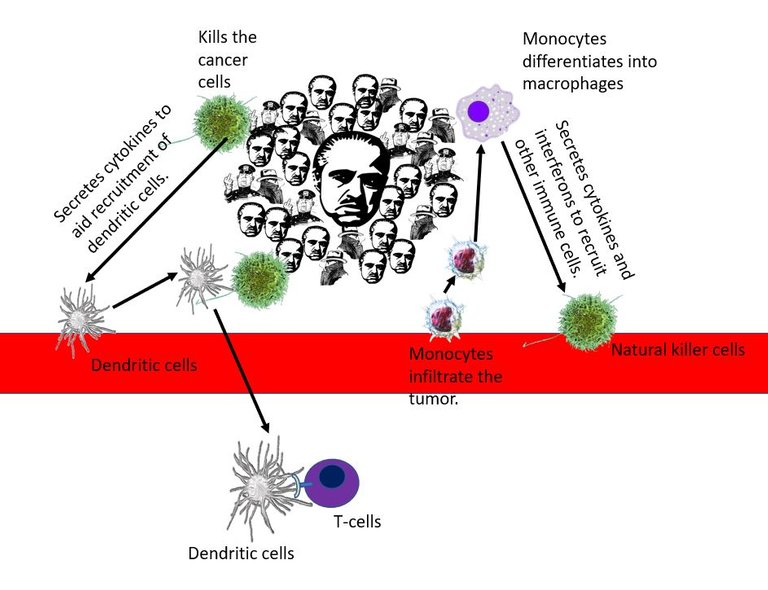
Monocytes from blood are attracted to signals coming from the tumour microenvironment. They migrate and infiltrate the tumour. The monocytes then differentiate into macrophages in the tumour. Macrophages are phagocytic immune cells, which means they master eating up the foreign and damaged cells and molecules in the tissue. They also detect the state of tissue-damaged cells and molecules secreted by cells in the tissue, which are abundant in a tumour microenvironment.
Illustrated using Natural killer by NIAID | CC BY 2.0, macrophage by A. Rad, Mikael Häggström, Spacebirdy, CC BY-SA 4.0, Monocyte by
BruceBlaus | CC BY 3.0, Dendritic cell and T cell by scienceblocks, Godfather, mafia and cop from Pixabay
This triggers them to secrete signals to recruit other immune cells such as dendritic cells and natural killer cells. Natural killer cells can either directly identify and kill the cancer cells or secrete more signals that recruit more dendritic cells. Dendritic cells on the other hand specialize in eating up the stuff in the tumour microenvironment and presenting the samples to T cells which can then get trained to identify and kill the tumour cells. Well, at least that is what the anti-tumour immune cells are supposed to do. But that's not what the immune cells that have changed sides do. Pro-tumour guys are inefficient in mounting a good fight against tumour cells. And, they even help the tumour to grow and spread.
Illustrated by @scienceblocks
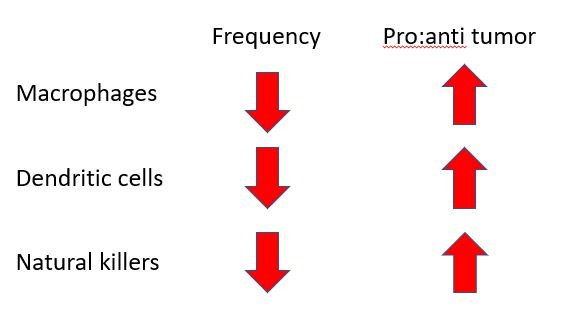
Anyhow, how do the macrophages isolated from tumours in germ-free mice look like? Well, first of all, there was a reduced frequency macrophage in tumours of germ-free mice. And then the macrophages that were there were majorly pro-tumour. In line with this, there was also a reduced frequency of dendritic cells and natural killer cells. And those dendritic and NK cells that were there seemed inefficient or mellowed down anti-tumour response.
Don't abuse antibiotics!
Image taken from pxhere | CC0
And no! This was not just some artefact of growing up in a completely sterile environment. You can feed the regular mice some antibiotics, and kill the healthy bacteria that they have. The tumours implanted in these mice on antibiotics showed similar characteristics for macrophages, dendritic cells and natural killer cells, as it was seen in germ-free mice. So perhaps one more good reason to stop abusing antibiotics without the advice of your health care professional, right?
Where, when and how?
The question however remains that how are bacteria in the gut controlling all the shit that goes on with the tumour associated immune cells. And it wasn't like the total number of these immune cells were reduced in the body. The monocytes (precursor cells to macrophages), the dendritic cells and NK cells numbers were fine everywhere else in the body. So why were they less in the tumours of germ-free mice? And why majorly pro-tumour immune cells have been homing in the tumours of the germ-free mice and of the mice on antibiotics?
Illustrated using Gut microbiota by DBCLS) | CC BY 4.0
Cycle di-AMP by Olayemiajao | CC0 1.0, IFNB by Nevit Dilmen | CC BY-SA 3.0, Natural killer by NIAID | CC BY 2.0
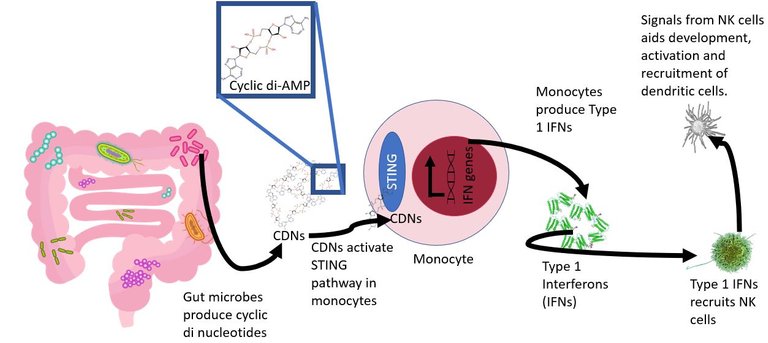
Turns out some bacteria in the gut are required to mentor the immune cells just right. For instance, in this paper they further show that some microbes are pro in delivering these immune cells, esp monocytes, signals called STING agonists. The STING agonists are molecular signals that instruct the receptive cells to produce type 1 interferons. Yeah, the same interferons the cells produce in response to viral infections. The type 1 interferon along with other signals, help in the recruitment of the natural killer cells and activate them just right to kill the tumour cells. The NK cells also secrete cytokines such as interferon-gamma which helps in recruiting and activating dendritic cells. And guess what was lacking on tumours of mice with fucked up or no gut microbes at all? Yeah, the type 1 interferons and Interferon stimulated genes. In line with this the genetically engineered mice incapable to recognize the STING signals or producing type 1 interferons, show a similar profile of the immune cells as seen in the germ-free mice. Furthermore, artificially giving cyclic dinucleotides ( a well-known class of STING agonists) to the mice, can help rescue the balance and produce more antitumour immune cells in germ-free mice.
Eat healthily and keep the gut bacteria happy!
In line with studies showing that some kinds of diets increase the risk of cancer, this study also found that the western diet (a diet rich in sugars and fats) is worse when it comes to cancer. It changes the microbiome in a way that either supports the pro-tumour ideology of immune cells or at least does nothing to stop them. On the other hand, mice that were given a high fibre diet plan developed a microbiome that promoted anti-tumour sentiments in the immune cells. The question however is which bacteria of all the bacteria that live in our gut is an efficient promoter of anti-tumour sentiments.
Which bacteria?

An image of a fixed bacterial cells stained with uranyl acetate, taken by me under TEM.
For this, the authors sequenced and identified the differences in the kind of bacteria that home the gut of mice fed on a high fibre diet. They identified a microbe called Akkermansia muciniphila. This microbe has been previously correlated with a better response to cancer therapy. In this paper, the authors show that A. muciniphila produces a STING agonist called cyclic-di-adenosine monophosphate, which is a potent stimulator of type 1 interferon production by monocytes. If you home the gut of germ-free mice with this bacteria alone, it was sufficient to skew the profile towards antitumor.
Increasing the efficacy of anticancer therapies
Now if you are thinking that if we have a healthy diet and don't abuse antibiotics we will not get a tumour? Well, carcinogenesis depends on a lot of factors. So, yes a healthy lifestyle, in general, would reduce the probability. But, there is also something else you can do with this knowledge. You can use it to make cancer therapy more effective.
For instance, mice in which interferon signalling in monocytes was effectively induced by diet or microbiota or direct stimulation responded better to anti-cancer drugs.
This was further supported by the evidence from human clinical trials. It has been shown in humans that the kind of microbes in the gut affect the outcome of some anticancer therapies. For instance, over here authors show that patients who don't respond well to immune checkpoint blockade (ICB) therapy, have type 1 interferon, cytokine and immune cell signatures similar to those of their germ-free mice. While the responders show the opposite trends.
Shit to the rescue!
Illustrated using public domain's poop, plate from MS copyright free icons, Gut microbiota by DBCLS) | CC BY 4.0, silhouette and gut from pixabay.
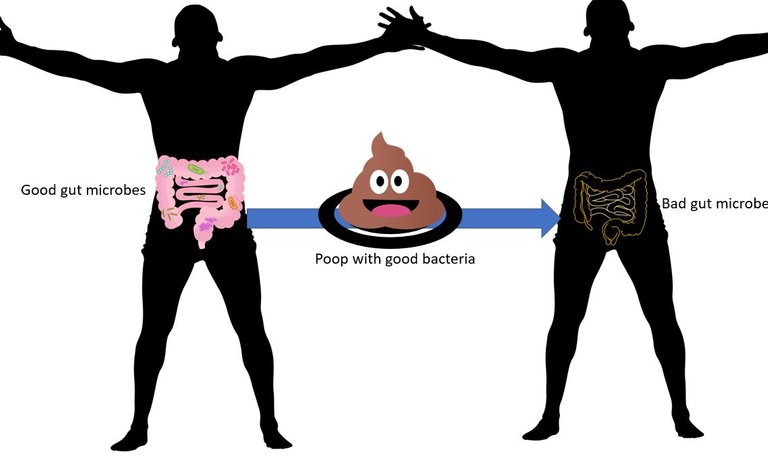
Also, you can take microbes from the faeces of responders and non-responders and implant them into germ-free mice. Doing this showed that mice that received microbes from responders show reduced tumour growth than mice that get it from non-responders.
Now, what if you take these faecal microbes from responders and give them to say melanoma patients receiving ICB instead, in a clinical trial setting? Turns out that the faecal microbiota transplant results in desired immune profile. The positive response was also correlated with the abundance of Akkermansia muciniphila in the guts of these patients after receiving the transplant.
Got more work to do!
However, it is to be noted (and the authors address this too) that we still don't know how and where the STING agonists (cyclic dinucleotides) interact with the monocytes that migrate to the tumour. The authors could not detect enough levels of these molecules in the tumour at least. Maybe it is a cascade that starts in the gut tissue itself and in lymph nodes associated with the gut. But it remains to be figured out.
Things to look forward to
Nevertheless, this study opens up a lot of novel anticancer therapeutic strategies to be explored. Some of which are already being studied. I mean forget the drugs, the fact the high fibre diet can enrich the good bacteria should be a good enough reason to look into the dietary intake of cancer patients. Also, it's never a bad idea to include some fibre in your diet. I mean why wait for cancer when good lifestyle practices can reduce the risk for it anyway.
Another interesting therapeutic approach other than using just diet or the faecal transplant is to directly use pro and prebiotics that results in favourable gut microbes. One can also think of developing pills to directly deliver cyclic dinucleotides or other synthetic STING agonists. Maybe combining the ICB with type 1 interferon therapy could come in handy. And if we can figure out how and where and how the interaction with the immune cells is happening we can figure out ways to directly skew the immune cells to anti-tumour kinds.


References
The Role of Diet in Cancer Prevention and Chemotherapy Efficacy
[Cancer and diet: What’s the connection?](https://www.health.harvard.edu/cancer/cancer-and-diet-whats-the-connection
Influence of diet on the gut microbiome and implications for human health
Host traits, lifestyle and environment are associated with human skin bacteria
The evil plan of cancer to hijack wound healing machinery
Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment
- [Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment]
You can reach out to me on discord if you are in stemsocial discord. Or you can even send me a DM. My discord handle is the same as my hive - @scienceblocks. You can also ping me on my telegram handle: @UncertainHeisenberg. Or follow me on Twitter: @scienceblocks1



It was a good read. !discovery 35
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Delegate to us for passive income. Check our 80% fee-back Program
Your content has been voted as a part of Encouragement program. Keep up the good work!
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for Proposal
Delegate HP and earn more
This is rich and easy to understand. Over and over again, facts keep emerging that if you genuinely watch what you eat in a healthy way, your chances of coming down with pathogenic and nonpathogenic diseases become significantly reduced. Of particular interest is the role of gut microbes in mitigating cancer. It opens up an expanse area of research that might lead to interesting discoveries, including possible drugs against cancer.
Thanks for sharing such a well-researched and easy-to-understand post.
Indeed. A important talk we all need to have is what makes a healthy diet. I mean since the industrial revolution we have been so busy in our lives, and all kind of processes and yummy junk food items are so readily available. While I completely understand that it is difficult to survive without those. Like if not that processed table sugar, what am I supposed to put in my milk coffee that makes it equally tasty? Apart from being yucky I dont even know what all effects would artificial sweetners such as sucralose have on the gut bacteria! And no, I dont think even my counterpart in any of the possible parallel universes would be using stavia!
Nevertheless, we can be conscious and mindful about the usage. And, at least make our diets better in other aspects. For instance, increase fiber intake.
But that being said one has to be careful in thinking of diet as a magic bullet as well. I mean it makes a lot of difference. But there are other factors - mental stress, sleep, environmental pollutants etc that we hardly talk about. They also affect the gut microbes.
Then guy microbes can be shaped by our genetics. Even the presence of tumor in the body changes the abundance of different bacteria. It's a two way talk between the human cells and bacterial cells. But yes, we can't change our genetics or disease once it's developed, but we can sure try our best with diet and having a good mental health.
Wow, Thank you for wonderful articel!
Thank you. Glad you liked it.🙂
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support. Using the STEMsocial app could yield even more supporti next time.