Atoms are mostly empty |Details with proof | Science
An atom consists of electrons, protons and neutrons. An atom is 100,000 times smaller than an angstrom. Its size is about 10-10 meters. The mass of a proton is slightly less than that of a neutron, which is 1 amu. The size of a proton is 0.84×10−15 to 0.87×10−15 m. The nucleus of an atom is made up of protons and neutrons Electron resides outside the nucleus and revolves around it. There is a lot of empty space between the nucleus and the electrons of an atom. If an atom were a football field, the nucleus of the atom would be like a marble located in the center of the field. Then you understand how much empty space there is in an atom.
Rutherford conducted an experiment with alpha α particles known as Rutherford's Alpha Scattering Experiment. This experiment shows us that there are many empty spaces between atoms.
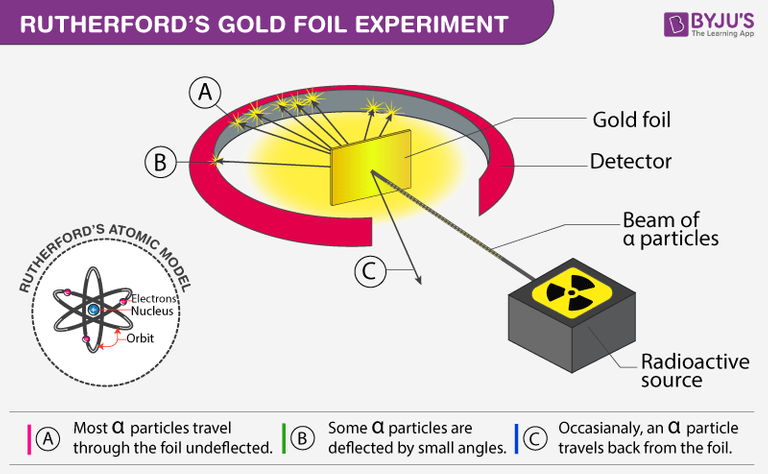
Rutherford performed this experiment by beaming high-energy α-particles from a radioactive source onto a thin sheet of gold, and placing a fluorescent zinc sulfide screen around the gold foil to observe the deflection of the α-particles, which emits a flash of light as the alpha particles fall on it.
After dropping each of the alpha particles onto the thin gold foil, they flowed through the atoms and hit the fluorescent screen, and the resulting flash of light on the screen was observed by Rutherford through a microscope attached to the back of the screen.
He observed that most alpha particles went straight through the gold foil. Some alpha particles deviate slightly from their path to interact with positively charged particles. Since the nucleus is a positively charged particle, the alpha particle interacts with it and changes its direction.Other alpha particles were scattered at large angles, and fewer alpha particles were returning to the source because the particles changed paths, interacting with the nucleus and traveling straight. In this regard Ratheford said that
"It was almost as unbelievable as if you had fired a 15-inch shell at a piece of tissue paper and it came back and hit you." _ Ratheford source
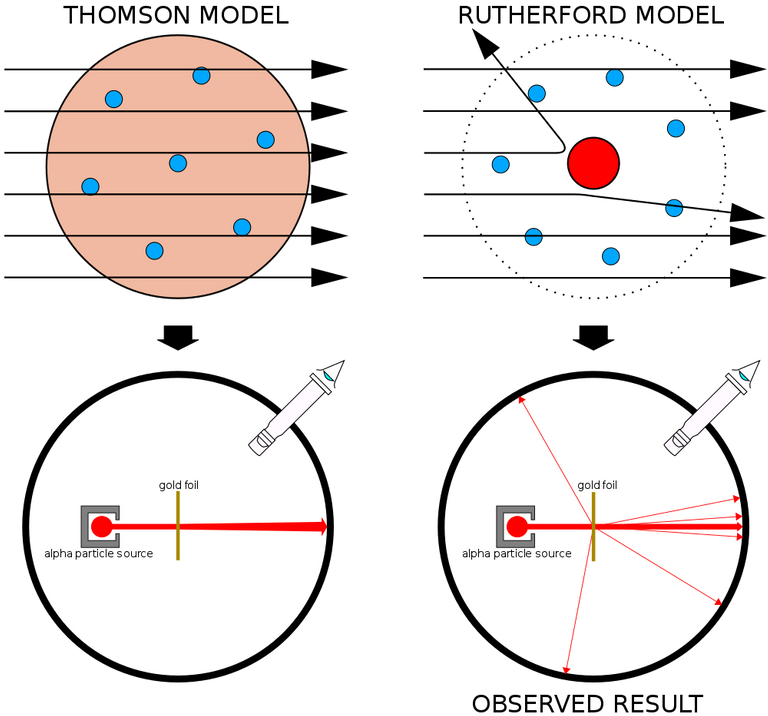
It was then assumed that a positively charged and relatively heavy particle resides in the nucleus and is the reservoir of repulsive energy. The negative electrons that electrically balance the positive charge move in circular orbits around the nucleus. At that time the atom was compared to our solar system. It was thought that the same way that the planets revolve around the Sun, the electrons revolve around the nucleus.
Rutherford realized that most of the space in our solar system is empty, just as the space in these atoms is mostly empty. So the atom was not able to resist the alpha particles. That is, the experiment was able to easily prove that there is a lot of empty space inside all the atoms around us.
If we turn our attention to a neutron star or a black hole, we can understand how much empty space is inside an atom. To make the matter more clear, please read the following texts carefully.
Black holes are cosmic objects of intense gravity from which even light cannot escape. How is it possible for a black hole with so much gravitational force to form a black hole through the death of massive stars? Once a giant star runs out of internal thermonuclear fuel at its core. Then it cools down. As the temperature decreases, its pressure decreases and it begins to collapse towards its center due to its gravitational force. This process is very fast and takes less time which is only fifteen. Think about what could happen if such a big star collapses towards its center in such a short time? It has a boon effect that causes a huge power full shock wave. This time the outer part of the star then explodes in what is called a supernova explosion.
Now suppose that a giant star went supernova and became a neutron star. We know that a neutron star supernova was preceded by a massive star with a total mass between 10 and 25 solar masses. Neutron stars are the smallest and densest class after black holes. Although it was a massive star, after the supernova the neutron star had a radius of 10 km and a mass of about 1.4 solar masses. Now think that a star many times bigger than our Sun becomes only 10 kilometers in radius as a result of super nova. Then see how many times the density of this star is increasing.
A spoonful of material from a neutron star can have the same mass as a giant mountain on Earth. How is such compression of matter possible? Because the mass of the neutron star is so intense, the empty space between the atoms decreases, which makes it possible for matter to shrink so much. If there was empty space in matter then neutron stars and black holes would not have formed. You will be surprised to know that a black hole can be the size of an atom. Is it possible to calculate the number of billion trillion atoms in a giant star? If so many atoms occupy the space equal to one atom, then you can understand how much empty space there is in an atom. From all the above description it is evident that a large part of an atom is empty space.
References :
byjus
forbes
NASA
wikipedia
wikipedia
Thanks for reading
Best regards

Great post, thank you for your contribution!
!1UP
Click this banner to join "The Cartel" discord server to know more.
Thank you
You have received a 1UP from @kwskicky!
@stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.