Atoms and the structure of matter.
When talking about atoms, Dalton's name comes first. According to Dalton all matter is made of tiny indivisible particles, which are called atoms. That is, an atom is that part of matter that cannot be broken. As we divide a matter, it reaches a point where it can no longer be divided. This is the atom. All matter is made up of these atoms. But with the development of technology, the concept of atom has changed.
Many bricks are used to build a wall. Comparing these bricks with atoms helps us to understand what an atom is. Although the atom is not as big as a brick. In fact, atoms are so small that they cannot be seen with the naked eye. However, if we think of walls as matter, then the atom is a brick. It is said only for ease of understanding.
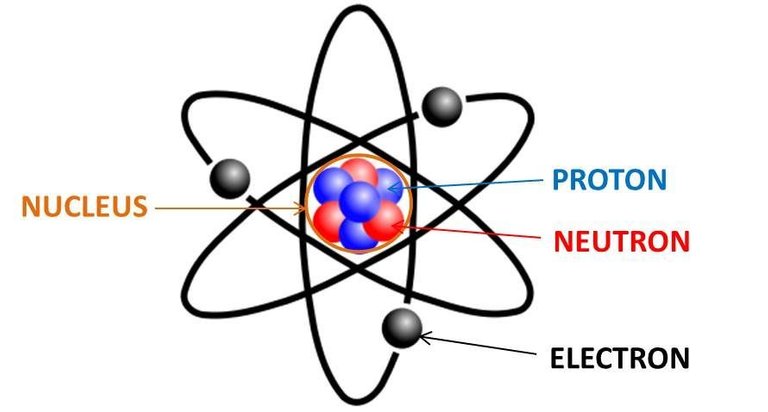
Along with the development of science and technology, we are introduced to the smallest particles of an atom. Now we know that atom consists of neutrons protons electrons. These particles have mass and charge. Proton and neutron have almost equal mass. The mass of an electron is thousands of times less than the mass of a proton. But electrons and protons carry equal and opposite charges even though their masses are so small and so large.
Along with the development of science and technology, we are introduced to the smallest particles of an atom. Now we know that atom consists of neutrons protons electrons. These particles have mass and charge. Proton and neutron have almost equal mass. The mass of an electron is thousands of times less than the mass of a proton. But electrons and protons carry equal and opposite charges even though their masses are so small and so large. A proton acquires a positive charge and an electron acquires a negative charge. A neutron is charge neutral
Protons and neutrons are held together in the center of the atom. The center of an atom is called the nucleus. Electrons reside outside the atom's nucleus and move around. This occurs for protons and electrons of opposite charge. Electrons revolve around the nucleus because protons have a positive charge and electrons have a negative charge.
We know that positive charge and negative charge attract each other. Protons and electrons also attract each other in the same way. This force of attraction holds the elements of an atom together. Since an atom has equal number of protons and electrons, it has no net charge. A proton has the same charge as an electron, so its total charge will be zero. But atoms that have extra electrons or no electrons have a net charge. If an atom has two electrons opposite to one proton, the two electrons will have twice the charge of the proton. If there are no electrons then only protons will have charge. That is why this type of atom has a net charge. These are called ions. Ions carry negative charge or positive charge. As a result they attract each other
To know more about the mass of atoms, it is necessary to know how atoms are measured. The unit of atomic mass is amus. The mass of an atom is very small, so its units are also small. One amus is one twelfth of the mass of one carbon 12. This is a small number expressed as 1.66 x 10'-27 kg.
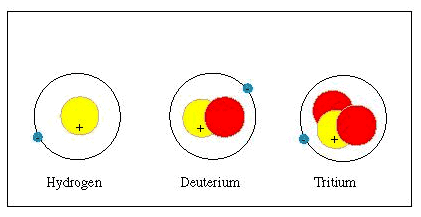
Isotopes of an element have different numbers of neutrons in their nucleus
All isotopes of one element have identical chemical properties. This means it is difficult to separate isotopes from each other by chemical processes. However, the physical properties of the isotopes, such as their masses, boiling points, and freezing points, are different. Isotopes can be most easily separated from each other using physical processes. source
A hydrogen atom that has a nucleus has a mass of 1.008 amu. A hydrogen atom that has two neutrons in its nucleus for a single proton has a mass of 2.014 amu. It is called deuterium. Again, the nucleus of a hydrogen atom has three neutrons for a single proton. Their mass is 3.016 amu. They are called tritium. All these isotopes of hydrogen have one electron each.
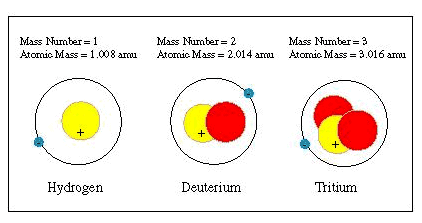
The sum of the number of protons and neutrons in the nucleus is the mass number of that element. Different isotopes of an element have different mass numbers because they have different numbers of neutrons in their atomic nuclei. Hydrogen is labeled as H1, deuterium as H2 which is clearly visible in the above diagram.
Atomic numbers are used to distinguish elements. The modern periodic table of elements shows the various elements arranged in increasing order of atomic number as shown in the figure below.
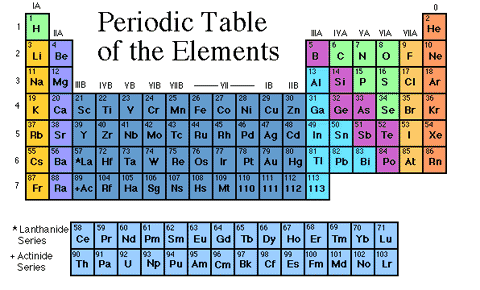
Scientists always use this periodic table. By referring to the atomic number, they know how many protons and neutrons are in the nucleus of that element. So this table is useful in several ways. Like helium 2 means a helium atom contains 2 protons. Gold has an atomic number of 79, which means it has 79 protons in its nucleus. There are currently 118 elements in the periodic table. Everyone from students to scientists use the periodic table
Source NASA 's website
Thanks for reading
Best regards

Congratulations @momins! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 200 posts.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Support the HiveBuzz project. Vote for our proposal!
The concept of atom is very important in all branches of sciences!
!1UP
Click this banner to join "The Cartel" discord server to know more.
Thank you
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
PIZZA Holders sent $PIZZA tips in this post's comments:
@curation-cartel(20/20) tipped @momins (x1)
Join us in Discord!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
A good basic introduction to the structure of an atom. When you think of the thousands of years that passed without a true understanding of the atom's structure, and then you think of how rapidly our understanding of the atom has progressed since Rutherford--Wow.
I've been reading @lemouth's blogs on physics and have learned about muons, quarks (don't understand all of it 😄) and other parts of the atom. Leaps and bounds, leaps and bounds. The sheer speed of it (given how long it took for the initial discoveries) makes my head spin.
Thanks for this overview of the atom's structure. It is very clearly described.
Human understanding of atoms has changed over the centuries. At first, atoms were explained based on theory alone. But with the development of technology people have learned to know the right things with evidence. But it may take many more years to gain advanced knowledge about atoms. Maybe we will learn more about atoms in the future
Thank you