THERMODYNAMICS APPLIED TO METABOLISM //Uses of ATP as an exchange currency.
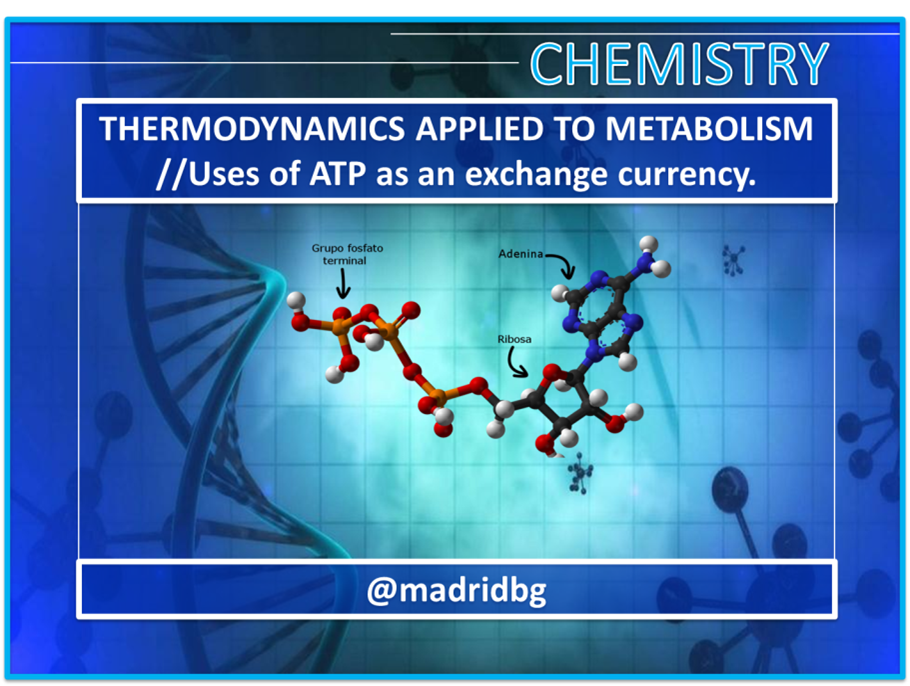
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of the #Hive platform, who share the same passion for scientific content oriented to chemical principles as the central idea of the phenomena that form our daily life. This issue is addressed to those readers who are part of the #Stemsocial community, who promote and support scientific topics on a daily basis. Therefore, the publication refers to the use of Adenosine Triphosphate (ATP) as an exchange currency in the different metabolic processes of our body, as well as the chemical principles present in such activity.
INTRODUCTION
At the metabolic level many chemical reactions are carried out, some catalyzed by enzymes, others perhaps not, so the human being as a whole we can call it as a ambulatory reaction, because our whole system, works using as energy currency the molecules of adenosine triphosphate (ATP), which behaves as raw material or main source of energy of our body, necessary for the development of the different functions of the organism are carried out.
Chemically, the ATP molecule stores all the necessary energy through the electrons of the phosphate group and participates in the different cellular processes that produce energy (glycolysis, Krebs cycle and oxidative phosphorylation) where ATP is invested to produce electron transport NADH (reduced nicotinamide adenine dinucleotide) and FADH2 (reduced flavin adenine dinucleotide) and obtain a net gain in the form of adenosine triphosphate.
The release of energy from ATP molecules lies in a literally simple process (ATP hydrolysis) where an ADP-ATP interaction takes place, i.e. a cleavage occurs in the molecule and the energy stored in the bonds is released, resulting in adenosine diphosphate (ADP) molecules that can be transformed into the starting molecule (ATP) in different metabolic environments. The energy obtained, enters the anabolic and catabolic system of our body and can be used in the synthesis of macromolecules such as proteins, DNA, RNA, among others.
Therefore, starting from the premise that the metabolic system of our body is complex, in this publication we will focus on the study of the thermodynamic processes present in protein synthesis and its interaction with ATP molecules, macromolecule that we will use to exemplify the use of adenosine triphosphate as an exchange currency.
THERMODYNAMIC FOUNDATION OF THEMATICS
In this section of the publication, we will instruct the reader in the handling of some key fundamentals associated with thermodynamics that allow us to understand protein synthesis and its interaction with ATP, aspects that we will develop below:
SPONTANEOUS SYSTEMS VERSUS NON-SPONONTANEOUS SYSTEMS
Thermodynamically, two types of systems can be presented which respond to the second law of thermodynamics, in reference to the spontaneous systems, we can define them as that process that does not need external intervention to be carried out, because it occurs under its own conditions, on the other hand the non-spontaneous systems, make reference to those that continuously need some external factor in order to function.
A practical example of these systems is the formation of iron oxides (Fe2O3) on the surface of iron materials, a process that occurs spontaneously until all metallic iron is converted to rust unless the necessary corrective measures are applied. On the other hand, the inverse process, refers to the obtaining of iron from the corresponding oxide, this does not take place spontaneously since the reaction does not proceed unless the necessary conditions are established, being these externally induced.

Fig. 2. Oxide formation as a spontaneous process. Author: pxhere
JOSIAH GIBBS AND THE CONCEPTUALIZATION OF FREE ENERGY
Josiah Willard Gibbs, was a physicist born February 11, 1839 in the United States of America, although his name is little known, he was one of the precursors of the foundation of some thermodynamic principles, among which stand out that related to the adaptation made on the concept of free energy.
The conceptualization made by Gibbs starts from the concept of the second law of thermodynamics, which states that:
"The amount of entropy of the universe tends to increase in time " [2].
Quantitatively, the principle stated above is determined by the interaction that the variables associated with the system under study have with its surroundings, which is sometimes difficult to predict and calculate since the type of interaction that may occur between the aforementioned variables is not known.
In this sense, Gibbs proposed a mathematical equation that responds to the second law of thermodynamics, where the variables are implicit and where the spontaneity of a reaction can be determined.

Equation 1. Gibbs arrangement in correspondence with the free energy. Author: @madridbg, through Power Point 2010. Adapted from McMURRY, (2008).
Where ΔG, represents the variation of the Gibbs energy, T, the temperature and ΔS universe the variation of the entropy in the universe. When analyzing the proposed equation, it can be observed that pressure and temperature have to be considered as constant variables and where the aspects related to the environment are not taken into account. In this particular, Gibbs states that:
1. ΔG < 0 The reaction is spontaneous in the direction in which the chemical equation has been written. 2. ΔG > 0 The reaction is non-spontaneous. The reaction is spontaneous in the opposite direction. 3. ΔG = 0 The system is in equilibrium. There is no net change. [1]
THERMODYNAMICS APPLIED TO LIVING THINGS
In order to instruct the reader on the applicability of thermodynamic processes, in the metabolism of living beings, it is necessary to conceptually address what is referred to as coupling reactions, which consists of producing a non-favorable reaction, from thermodynamically favorable reactions.
Fig. 3. Analogical representation of a coupling reaction. Author: sdvigger
Knowing that metabolic reactions are usually unfavorable according to the variation of energy and therefore we classify them as endergonic (energy absorbing) and exergonic (energy releasing). Therein lies the importance of coupling reactions, since through these, processes that were unlikely to be carried out very easily in biological systems, a practical example of this is represented by the enzymatic functioning which allows a large number of reactions considered as non-spontaneous to be carried out.
Metabolically, the synthesis of ATP comes from the transformation that our cell produces on glucose, a molecule extracted from the different foods we consume according to the following equation:
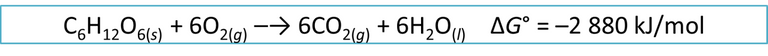
Equation 2. Obtaining energy from the breakdown of the glucose molecule Author: @madridbg, via Power Point 2010. Adapted from Chang (2010).
If we analyze the equation, we can see that the energy released is very high, so that in our body this reaction does not take place because we would be overloading our cells, as happens if our computer is plugged into a regulator based on 220 volts, even knowing that the equipment is trained to receive 110 volts, the results would be catastrophic.

Fig. 4. Food represents the raw material for obtaining glucose. Author: Einladung_zum_Essen
Therefore, our organism with the help of enzymes degrades the glucose molecule into several parts as mechanisms for releasing the energy contained in its bonds, because our system is efficient the energy released is used in the formation of ATP from ADP.
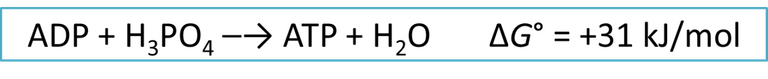
.Equation 3. Interaction between ADP-ATP and energy storage. Author: @madridbg, via Power Point 2010. Adapted from Petrucci (2003).
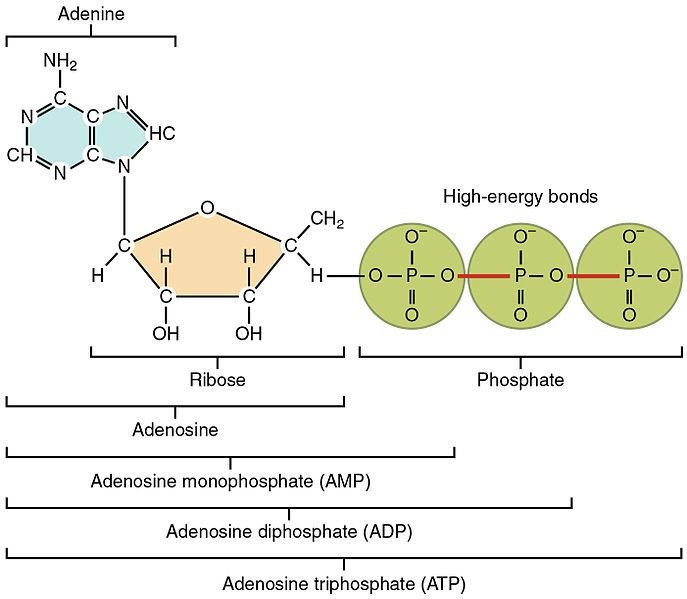
Fig. 5. Representation of the ATP molecule. Author: OpenStax College, (2013).
From the formation of ATP a certain amount of energy is stored which can be used in the synthesis of proteins since their formation is not spontaneous. It is necessary to remember that proteins, are large polymeric molecules structurally constituted by amino acids, where the variation of free energy is governed by the individual bonding of each of these substances.
A practical example of this process is seen in equation 4, where the binding of the amino acids alanine with glycine is a non-spontaneous process, but indispensable in the formation of the peptides that will give rise to the proteins, so it is necessary to resort to the energy of ATP and generate a coupling reaction which allows the union to develop normally.

Equation 4. Coupling reaction that allows the development of non-spontaneous reactions. Author: @madridbg, via Power Point 2010. Adapted from Chang (2010).
Therefore, at the metabolic level, every time a non-spontaneous reaction occurs, ATP must be called upon to inject the amount of energy necessary for the process to take place; the ATP consumed is restored by the same system through cellular respiration.
THEMATIC CONTRIBUTIONS
Through the subject matter, the reader was instructed on some aspects of thermodynamic interest, the fundamentals governing biochemistry in metabolic processes were also addressed and the import of adenosine triphosphate (ATP) in coupling reactions, essential for human life, was learned.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Gómez, T. (2005). Termodinámica. TECNUN. Notas de clase. Campus tecnológico de la Universidad de Navarra.
[3] Groel, N (2006). Generation of carbon dioxide by the reaction of an acid and a base Aquariological Silver Society . Article: Online Access
[4] Lavoie J y Col. (1995). La modulación de la termorresistencia celular y la estabilidad del filamento de actina acompaña a los cambios inducidos por la fosforilación en la estructura oligomérica de la proteína de choque térmico. Mol Cell Biol. 15. 505-16.
[5] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[6] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[7] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
HTTP is in use instead of HTTPS and no protocol redirection is in place. Be careful and do not enter sensitive information in that website as your data won't be encrypted.
It's also a good habit to always hover links before clicking them in order to see the actual link in the bottom-left corner of your browser.
This auto-reply is throttled 1/20 to reduce spam but if it still bothers you reply "OFF HTTP". Or reply REVIEW for manual review and whitelisting.
https://twitter.com/BGMadrid/status/1361722704311836675
#posh twitter
https://twitter.com/BGMadrid/status/1361722704311836675?s=20
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.