THE SYNTHESIS OF ORGANIC COMPOUNDS AS A SCIENTIFIC ART
Greetings and welcome dear readers to another installment associated with scientific knowledge and the theoretical-practical approach to science, specifically what makes mention to the study of organic compounds.
In this sense, by means of this delivery we will be approaching all the referents those consecutive reactions that are carried out as a measure to obtain a product of particular interest in which principles and established rules that generate the synthesis of the same one are followed.
Image courtesy of: Tomislav Jakupec
Therefore, throughout the previous publications we have made an approach through the family of hydrocarbons, specifically alkanes and alkenes, studying the different substitution reactions involved in the production and processing of alkenes, hence, in this opportunity we will specifically mention the family of alkynes, where it is necessary to establish that this type of hydrocarbons is characterized by presenting in its structure a triple carbon-carbon bond that gives it a higher degree of instauration than that of alkenes.
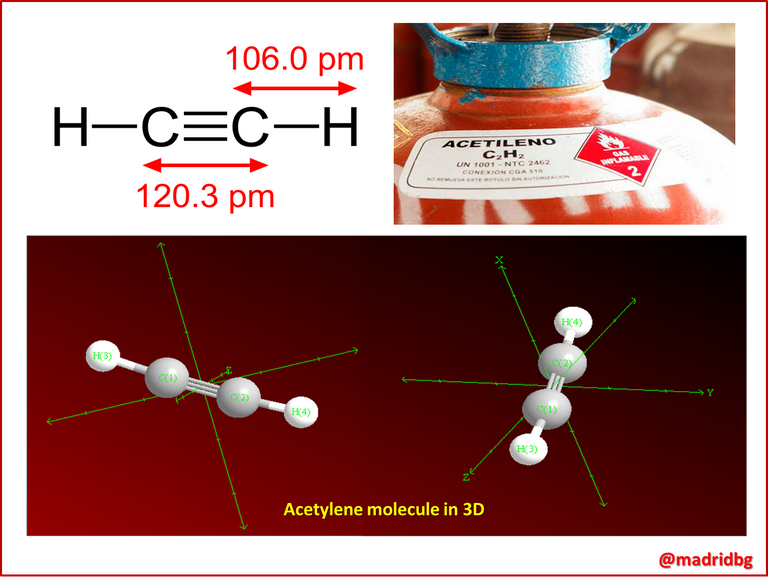
Thus, within this family, acetylene is the simplest alkylene and its use has been extended at industrial level, since it is used as raw material in the preparation of other compounds such as vinyl chlorides, acetaldehyde and even acetic acid itself, hence it is necessary that at experimental level the development and creation of new synthetic routes that are much more efficient and even mitigate the costs of reagents used is promoted.
In this sense, when we enter into the electronic structure of alkynes we have to know that an interaction is generated between the carbon atoms that share these bonds, they assume a hybridization of type (sp), in other words, these carbons when they interact form a bond angle of 180° along the axis perpendicular to the (2py) and (2pz) orbitals, characteristic that allows this type of bond to have an approximate energy of 835 kilojoule/mol, hence it is assumed that these bonds are much shorter than the single bonds that form alkanes and the double bonds that form alkenes, consequently it is necessary to implement much more energy to break this type of bonds.
In this sense, knowing this property is essential at the experimental level, hence when we talk about organic synthesis, we refer to all that planned and organized structural process that allows the execution of specific reactions to obtain a desired compound.
Based on the above, in the preparation process of alkynes, the route of alkenes is practically followed, so that they are prepared through the elimination of an alkyl halide with an excess of a strong base such as potassium hydroxide, processes that we can observe in the equations presented below.
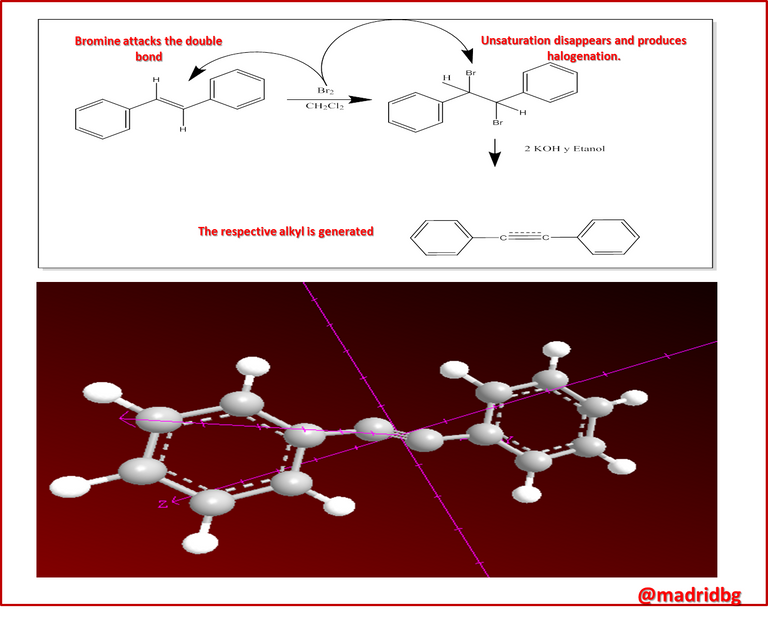
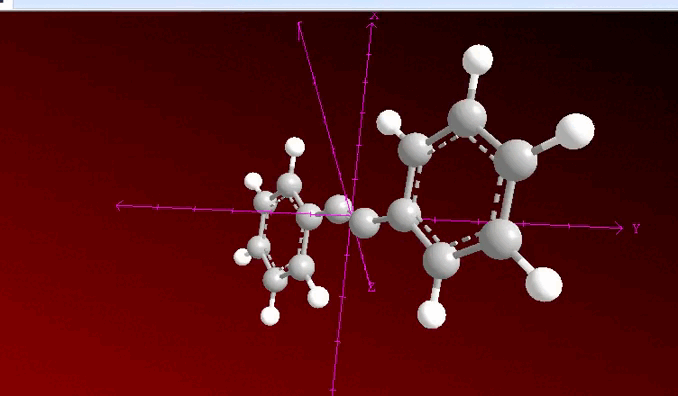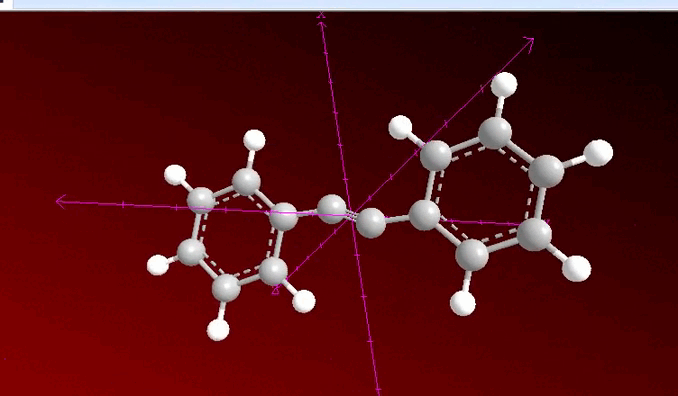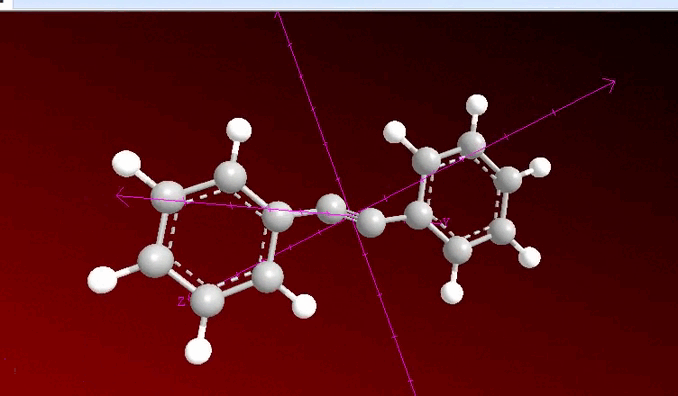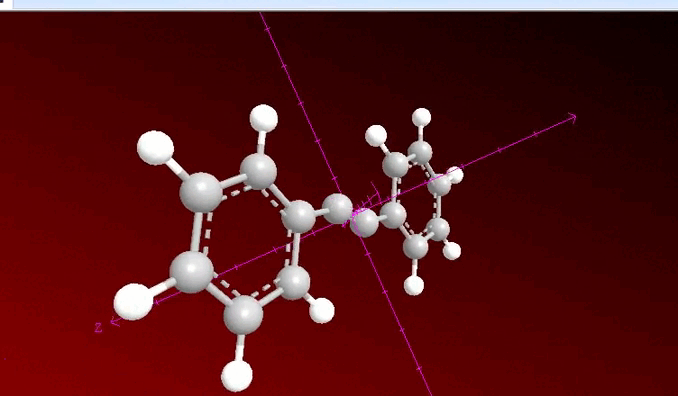
When analyzing the previous image, we can observe that a process of neighboring dihalogenation is being carried out, a reaction that is developed by adding bromine or chlorine as a species during the process and at this point, the general sequence of the process allows us to have a method to go from an alkene such as 1, 2 diphenylethylene to 2 diphenylacetylene which has been previously treated with a base such as potassium hydroxide.
From the above, we can establish that the family of alkynes can develop various reactions or chemical processes, among which we can highlight: addition and halogenation reactions, we can also subject the alkynes to a hydration process catalyzed with mercury, on the other hand we can trigger the hydroboration and oxidation, without leaving behind the processes of reduction of these compounds in much smaller molecules, so that there is diversity of treatment for this type of compounds and each of them can be used sequentially or alternately in the process of organic synthesis.
Now, the idea behind introducing this line of writing to the development of organic synthesis is directly related to the treatment and utility that is given to the molecules that are synthesized in a laboratory, an example of this is represented by the pharmacokinetic and pharmacodynamic work that is pursued at the level of the pharmaceutical industry, where organic molecules are designed and synthesized hoping that some are used through a treatment or have a direct and positive effect on the health of people.
On the other hand, the model of organic synthesis at the chemical industry level allows the generation of new synthetic routes and models that are more economical in terms of the elaboration and preparation of already known compounds, hence the intellectual process, planning and creative development allows scientists to describe in subjective terms, the elegance and beauty that an organic compound can exhibit.

Image courtesy of: Colin Behrens
Consequently, the process of organic synthesis aims to generate the ability to plan a sequence of synthesis that is feasible and for this it is necessary to know several variables within the organic reactions, in addition practical skills are required to chain the steps of this sequence, so that it seeks to alter specific locations within a molecule because it will be those places that function as a point of action or catalytic center to break or add atoms that increase or decrease the size of a compound.
To exemplify the above, let us suppose that we want to prepare octane from 1-pentane, when developing on paper and comparing both compounds we realize that 1-pentane lacks 5 carbon atoms and consequently there must be a reduction of the triple bond, i.e. we will go from an unsaturated compound to a saturated compound, so this is where the creative part of the scientist or the person in charge of developing this procedure begins.
In this case, using an alkyl halide such as 1-bromopropane, what we are doing with this reaction is allowing the addition of three carbon atoms to the compound and then reducing the product by means of a catalytic hydrogenation process and thus obtaining the expected product.
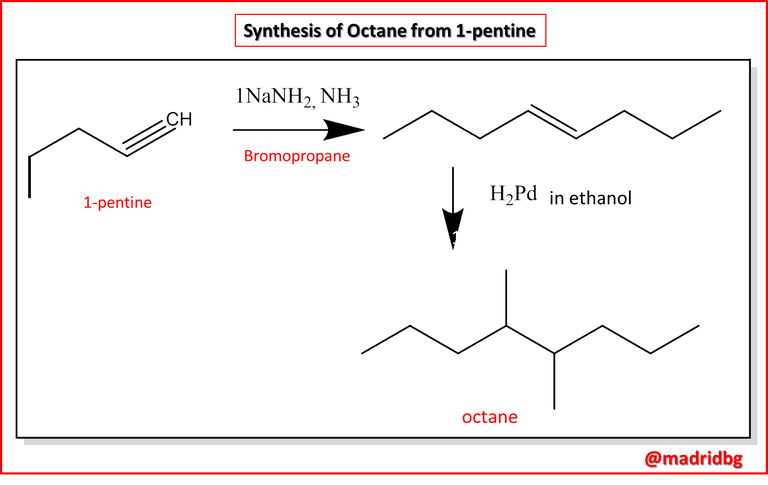
Undoubtedly the process is not as simple as mentioned here, because we must take care of variables such as amount of reagent, temperatures, pressure, amount of product we want to obtain, catalysts that accelerate or slow down the reaction and all this should be a subject that the analyst must handle to produce the desired compound, it should be noted that the route just mentioned is not practical economically speaking, because it is much more expensive the synthetic process than buying the octane that is obtained through a much simpler process perhaps extracted from hydrocarbons.
However, this does not imply that the synthesis process is not important, on the contrary, through this methodology it has been possible to obtain a large number of compounds known today and that allow to exert a direct action at pharmacological level for the treatment of diseases and the improvement of health in the human species.
In this sense, if we think that the natural process is complex, we do not even want to imagine the whole process or route that is followed to obtain molecules such as vitamin B12, so that if we make the respective review, we realize that it is very simple to go to a pharmacy and buy the drug in question, now it took dozens of years of research and studies for Robert Woodward in 1973 to culminate with his collaborators the synthesis of this spectacular molecule.
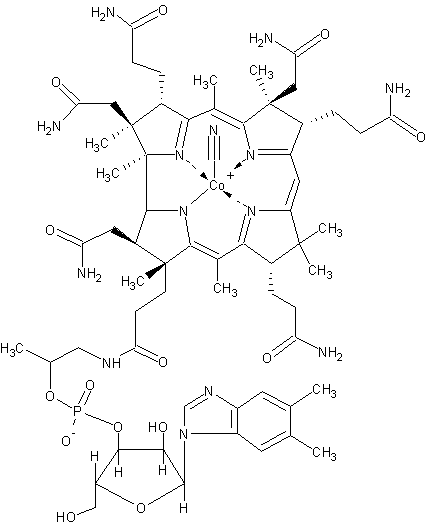
Representation of the structure of the vitamin B12 molecule. selfmade by Azazell0
So it is valid to ask why spend so much time and effort in the synthesis of a molecule that is very easily obtained through natural processes or sources? The answer to this question comes from the fact that it represents a challenge for human knowledge itself, where we move in function of solving a problem and at the same time we strive to obtain a useful product for our society, just as a mountaineer challenges himself to climb the highest mountain, chemists assume as a challenge the development of a process that allows us to provide a utility to our environment, hence through organic synthesis we prepare new substance, generate new processes, obtain new reactions and with this whole system we understand the better functioning of matter through our laboratory experiences.
BIBLIOGRAPHY CONSULTED
[1] Deanna Marcano - Gustavo Cabrera. Principles of Organic Synthesis. Book: Online Access
[2] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[3] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[4] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
1. The molecular models presented were designed by @madridbg using Chem3D and Chemdraw software.
2. For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/279290402/status/1620788116759134210
The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
molecule simulation is for sure a type of art!!
!1UP
You have received a 1UP from @gwajnberg!
@stem-curator, @neoxag-curator, @pal-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
I gifted $PIZZA slices here:
@curation-cartel(16/20) tipped @madridbg (x1)
Join us in Discord!