STEREOCHEMISTRY OF ORGANIC COMPOUNDS AND ITS IMPACT ON DRUG USE
Greetings and welcome dear readers, we continue with the scientific approach associated with organic compounds and in this issue we will be studying all those related to the stereochemical aspects of these molecules and which generates a substantial impact on the process of obtaining and manufacturing drugs. Thus, the study of this property is decisive when it comes to establishing whether a drug has effective pharmacodynamics and pharmacokinetics in the treatment of pathologies.
In this sense, and as has been constant throughout the scientific dissemination of this blog, these are contents that we usually share through the @Stemsocial community where topics related to science are curated and valued.
Image courtesy of: qimono
Therefore, before going into context it should be noted that throughout our daily lives, we have become familiar with terms such as left-handed or right-handed specifically for those who perform a daily action with their left hand or with their right hand respectively, although we should be aware that most of us do not usually think about them.
However, it is normal to assume that we have developed many more skills with one hand than with the other and an example of this can be seen when we practice baseball, since when it comes to pitching the person who is right-handed will obviously do it with his stronger hand which is the right, while at the time of catching the ball, we obviously develop greater qualities in the left hand and the opposite case occurs with left-handed people.
Now, these same principles are often present at the level of organic compounds and according to this, terms such as stereochemistry of a chemical compound usually appear, which is nothing more than that property that allows to establish that such compound has a non-superimposable mirror image, that is, if we place ourselves in front of the mirror and project our right hand, we will realize that the reflection is nothing more than the left hand so that our hands are non-superimposable mirror images.
This property, although for many it may seem insignificant, plays a crucial role in the main function of organic compounds and is a direct consequence of the tetrahedral stereochemistry of the carbon atom with a hybridization (sp3), a property that is decisive in most of the drugs and molecules of our organism, since it allows a process of molecular laterality of the same and in turn generates specific interactions between molecules, which are of great importance in biochemical terms.
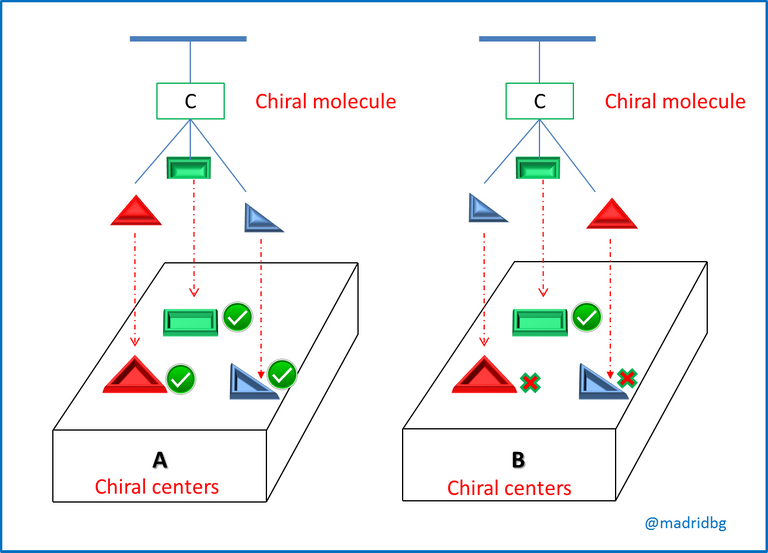
So in order to understand these theoretical constructs in depth, it is convenient to analyze the image presented below and as we can denote images 1 and 2 when we contrast them with the mirror, we realize that their mirror images are identical to those of the starting compound. However, when we observe image 3 we can see that this mirror image is totally different from the original image, so that within the composite 3 we can observe the relationship that exists between the left hand and the right hand, hence when we try to place one image over the other, we observe that they do not coincide which means that they are mirror images that do not overlap.
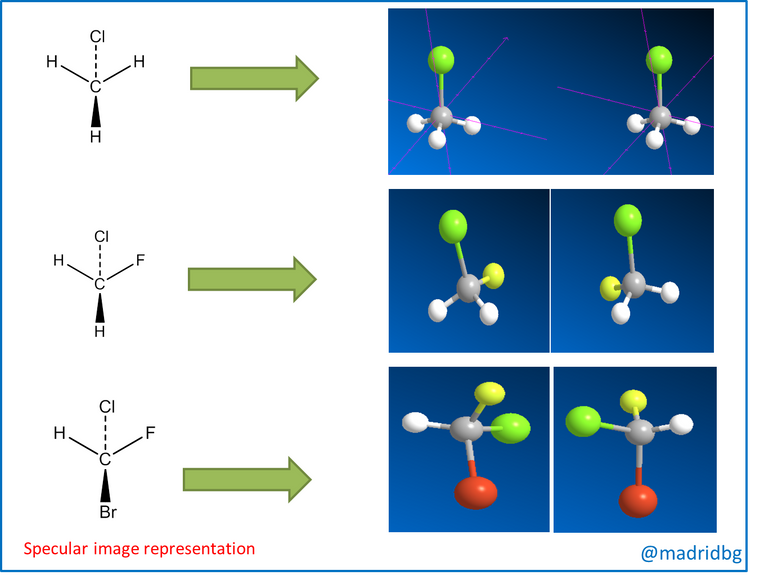
In this sense, at the level of organic molecules when the images are mirror images or cannot be superimposed, we say that we are in the presence of an enantiomer, that is to say a compound that is opposite to the starting material and these are produced when the carbon of the compound is tetrahedral, so that it has the capacity to bond with four different substituents, as we observe below.
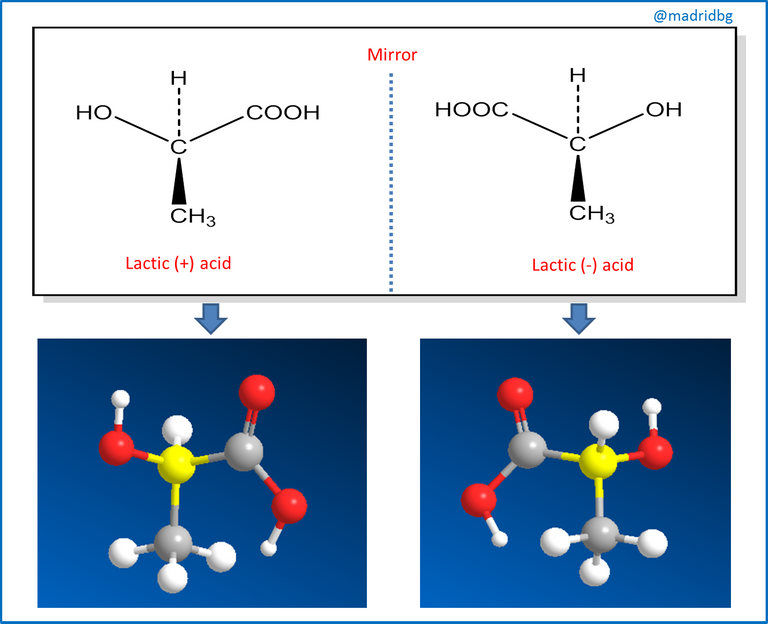
Therefore, and in order to understand the process of chirality of an organic compound, we can assume that when a plane of symmetry is present in the compound we say that the molecule is non-chiral and this study had its beginnings through the work of Jean Baptiste Biot, a French scientist who investigated the nature of light polarized in a plane, In his procedure, Biot passed a beam of ordinary light through an apparatus or polarizer, so that by directing the beam of light in a plane and coming into contact with a solution of the organic substance under study, it could turn the beam of light to the left or to the right and when this happened we were in the presence of an optically active molecule.
So that those molecules that turn the plane of light to the left, we say that they are levorotatory, while those that turn it to the right, we say that they are dextrorotatory and consequently this property is the one that has to be evaluated in the synthesis of organic compounds, mainly used in the preparation of medicines and in those that start as a compound of natural origin, since this optical activity allows us relevant information about the behavior of the active principle and the compound under study.
In this sense, in organic terms and according to the optical activity we can find compounds that are considered as diastereoisomers, racemic mixture or mesos compounds and these are terms that we will address in depth in future publications, however, it is necessary to remember that those compounds that have the same amount of atoms, the same chemical formula, but differ in their structure or spatial organization are considered as isomers.
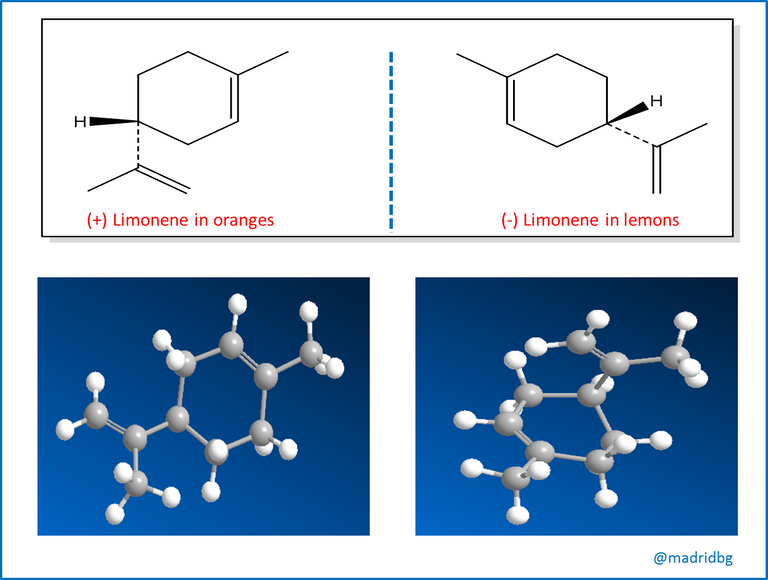
At this point, stereochemistry and isomers usually occur in nature, although the different enantiomers of a chiral molecule have the same physical properties they usually have different biological properties and a particular example is the one observed with the compound limonene, which has an orange odor when it is able to turn the plane of polarized light to the right, while the one that does it to the left has a characteristic smell of lemons.
Another example that we can use is the case of fluoxetine which is presented as a rasemic mixture implemented at clinical level as an antidepressant of extraordinary efficacy, however, this drug does not generate any effect on migraine and the strange thing is that the pure enantiomer (s), acts extremely effectively on this pathology and its activity and clinical potency is currently being evaluated in other medical areas.
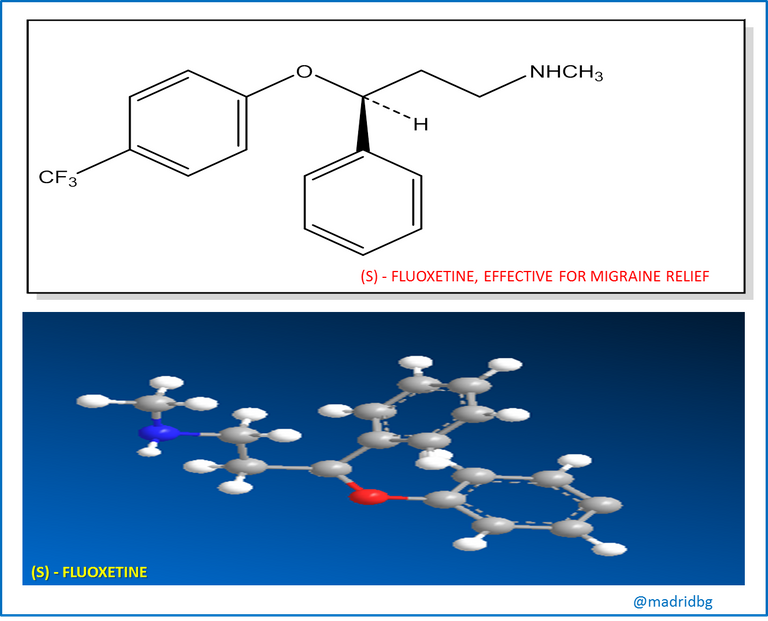
At this point, it is interesting to ask why stereoisomers, despite having the same number of atoms, have different biological properties? And in answer to this question, we must assume that for a compound to exert a biological action, the chiral molecule in question must enter a chiral receptor in a certain place, as our hand does in a glove, but we all know that our right hand will only enter a right glove, so that stereoisomers can only enter a receptor that has the right complementary form to it and any other stereoisomer will not fit in the best way and consequently the interaction between a chiral molecule and the receptor is what allows the biological activity within the organic compound.
At this point, it is necessary to understand that all those drugs that come from natural sources either directly or through chemical modifications, are usually chiral and are generally only found in the form of an enantiomer and not as a racemic mixture and an example of this is represented by penicillin in which this antibiotic is only present in one of its enantiomers and the other is not present or exists naturally, but it can be synthesized at the laboratory level.
However, it should be noted that unlike drugs obtained from natural sources, those that are prepared at laboratory level can be chiral and may or may not have an optical activity or can be produced and sold as a racemic mixture, an example of this is represented by ibuprofen where the enantiomer (S) has analgesic and anti-inflammatory activity, while its enantiomer (R) is inactive, although when it enters our organism it has the capacity to convert slowly into its isomer (S), a form that is clinically active or useful, which is why the formulas that are sold as drugs are usually presented as racemic mixtures of this compound.
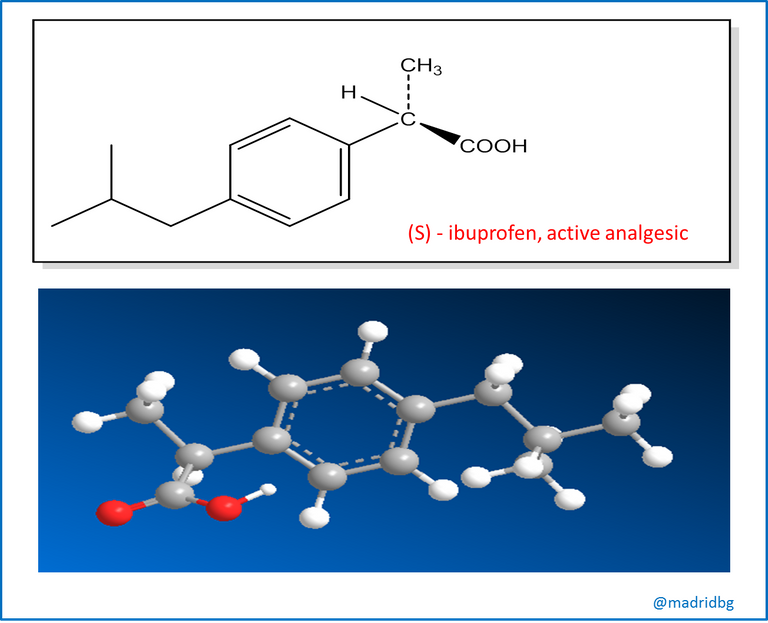
However, the process of synthesizing and administering an enantiomer that does not serve a particular function represents a waste from a chemical point of view, Likewise, evidence has been obtained of enantiomers that have caused negative effects on health and that can alter and affect the organism's capacity to use the correct enantiomer. To avoid these problems, pharmaceutical companies have begun to venture into a new method called enantioselective synthesis, a procedure that allows the preparation of only one enantiomer of the specific compound, and obviously it will be the one that fulfills a pharmacological function in the organism.
So again we can see how scientific advances have allowed us to scrutinize and evaluate the true functioning of chemical compounds, giving them the opportunity to exert a direct and useful action on our organism.
BIBLIOGRAPHY CONSULTED
[1] Navarro and collaborators Effect of isomers in clinical practice.Artículo: Acceso Online
[2] Fernanda Saldívar-González Drug discovery and development: a computational approach.Artículo: Acceso Online
[3] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
1. The molecular models presented were designed by @madridbg using Chem3D and Chemdraw software.
2. For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/279290402/status/1623333118940794881
The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.