PERICYCLIC REACTIONS AND THEIR IMPACT ON VITAMIN D CONFORMATION
Greetings and welcome dear constituents, back with the approach associated with scientific knowledge, in this installment we will be sharing information associated with pericyclic reactions that undergo some compounds at the organic level and that allow to generate various reactions that have been very useful for both biological processes and synthetic processes.
So as it has been constant throughout all this time that we have made life in the #Hive platform, we usually share this knowledge through the community of @Stemsocial leaders in the promotion and valuation of science.

Image courtesy of: Henryk Niestrój
Based on the above, it is necessary to understand that most of the reactions at the organic level start from a common principle and it has to do with the reactivity of those polar compounds in which normally the nucleophiles are able to donate electrons to an electrophile with which a new bond is established, we can also find reactions that are executed through the mechanism of free radicals and at this point each of the reactants yield their electron for the formation of the respective new bond.
However, if we enter into the study of pericyclic reactions we realize that the information about this is very reduced, so it is necessary to establish that this type of reactions occurs through a concerted process through a transition state of a cycle and when we mention the term concerted, we are assuming that all changes in the bonds are executed at the same time and in a single stage so that in this type of process no intermediary compounds are developed.
At this point, the pericyclic reactions can be classified into three types which respond to electrocyclic reactions, cycloaddition processes and sigmatropic rearrangement reactions, however, for technical reasons throughout this divulgation space, we will be mentioning the electrocyclic reactions which will be the common denominator where we will focus our study bases.
Therefore, and starting from the above, it is necessary to establish that the electrocyclic reactions are cyclic processes that include a process of cyclization of a conjugated polyene, at this point the bonds that form the structure, break and form a new bond which is responsible for generating the corresponding cycle, so that through the cyclization of a conjugated polyene can be obtained several cycles that will lead to the formation of a new compound.
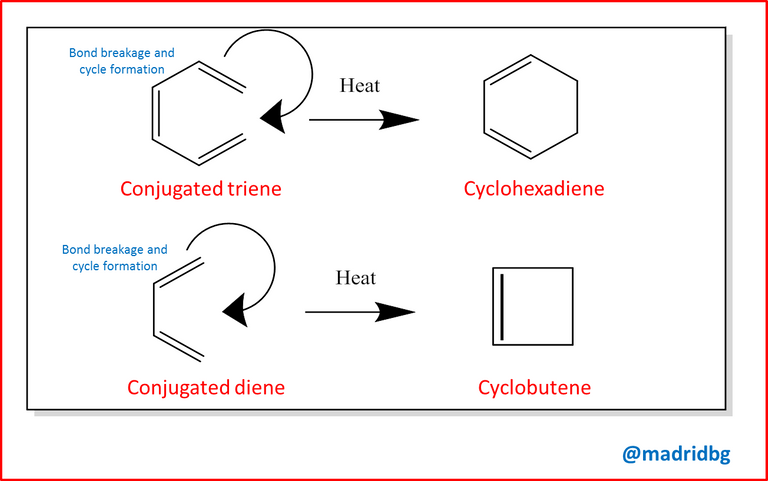
To understand this system in detail, let us analyze the previous image, in it we can observe how a conjugated sled in this case the 1, 3, 5 hexatriene when subjected to a heating process, the respective cyclization is formed and at this point the π bonds of the double bonds belonging to carbon 4 and carbon 5 are broken and give rise to the cyclohexadiene closed structure that has originated through a process or an electrocyclic reaction.
The same occurs with the compound 1, 3 butadiene which when subjected to temperature variations, again the π bonds break and generate the respective cyclobutene, so that when we go into the procedure we can observe that one of the most outstanding characteristics in this type of process, corresponds to the stereochemistry that presents the molecules in question since the structures can vary at a spatial and conformational level through their cis and trans structures concepts that we introduced in previous publications.
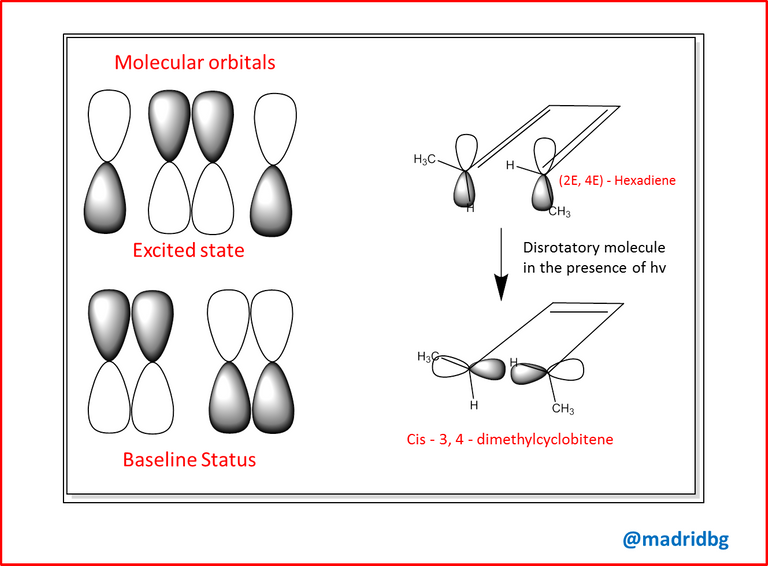
Consequently, at the biological level the electrocyclic reactions play a fundamental role but especially those that are activated by photochemical process, so that it is able to adopt a stereochemical organization different from those that are generated when subjected to a direct thermal process, as the previous example, hence the ultraviolet irradiation of a polyene, is capable of causing the excitation of an electron that is in basal state, which generates a change in the symmetry of the molecular orbitals of higher and lower occupancy respectively.
Consequently, photochemical processes alter the stereochemistry of the reaction so that it becomes necessary to understand concepts associated with photochemical cyclization where a conrotatory (opposite side orbitals) and disrotatory (equal side orbitals) stereochemistry is assumed instead of the classical cis and trans that we have worked on previously.
Based on the above, we analyze the behavior of pericyclic reactions at the biological level, specifically with regard to the synthesis of vitamin D also known as vitamin containing the sun's rays, consequently we must assume that it is a pericyclic reaction that is executed in the middle of a photochemical process.
So vitamin D, which was discovered in 1918 is part of a general name for two compounds that are linked or related, in the first instance we have cholecalciferol vitamin D3 and ergocalciferol vitamin D2, both have steroidal properties and differ only in the nature of the hydrocarbon chain that is directly attached to the 5-membered ring.
So that cholecalciferol comes from all dairy products and fish, while ergocalciferol we can get it in some vegetables, both have the function of regulating the process of bone calcification and increase intestinal absorption of calcium, which is why when there is an adequate concentration of vitamin D, Obviously, if the concentration of the vitamin is low, the absorption of calcium decreases by more than 10% and from here on in children the absence or deficiency of this vitamin produces diseases such as rickets.
At this point, it is necessary that we can understand that at food level there is neither vitamin D2 nor D3, what is present are the precursor molecules, referring to 7-dehydrocholesterol and ergosterol respectively, however, these molecules when they enter in the presence of sunlight, both become active vitamins under the skin, hence the nickname of sunlight vitamin.
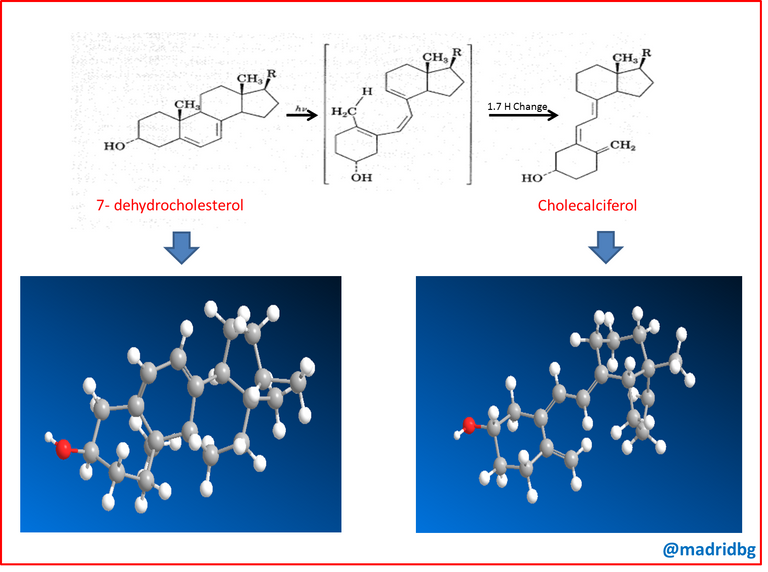
We must also understand that pericyclic reactions are unusual in living organisms and the photochemical synthesis of vitamin D is one of the few well-studied examples, so that the reaction proceeds in two steps, first an electrocyclic ring opening of a cyclohexadiene forming a hexatriene, followed by a hydrogen displacement forming a hexatriene, followed by a hydrogen shift that results in the formation of an isomeric hexatriene and at this point is supported by a metabolic process carried out in the liver and kidney which is able to induce the hydroxyl groups to give the active form of vitamin D.
Consequently, it is a complex process, which we can know from the advances in science, specifically the advances in chemistry and its importance in the development of life.
BIBLIOGRAPHY CONSULTED
[1] PERICYCLIC REACTIONS IN SYNTHESIS.Artículo: Acceso Online
[2] Abián Díaz Díaz Pericyclic Reactions in Domino Processes.Artículo: Acceso Online
[3] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
1. The molecular models presented were designed by @madridbg using Chem3D and Chemdraw software.
2. For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/279290402/status/1622074781959954433
The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Su post ha sido valorado por @ramonycajal
Gracias por el apoyo estimado @ramonycajal, como siempre atento en la valoración de los contenidos científicos.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thanks for your support friends of @stemsocial
Congratulations @madridbg! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 150000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
Support the HiveBuzz project. Vote for our proposal!
Thank you @hivebuzz friends for securing each of our accounts.
You're welcome @madridbg.
If you want us to continue keeping track of your achievements, we need your help!
All you need to do is to click on the "support" button on our proposal page: https://peakd.com/proposals/248. It won't cost you anything!
Thank you.
Very Interesting
!1UP
Thanks for your support friends
You have received a 1UP from @gwajnberg!
@stem-curator, @neoxag-curator, @pal-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
I gifted $PIZZA slices here:
@curation-cartel(3/20) tipped @madridbg (x1)
Please vote for pizza.witness!