LIQUID CRYSTALS // Action of intermolecular forces on the behavior of substances.
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome back to this space of chemistry in context, where every week we contribute from the communities of @stemsocial, scientific content under a flexible structure, which allows the proper understanding of chemical principles that usually have to be considered as complex and worthless.
In this sense, in this opportunity we will address the intermolecular forces exhibited by chemical molecules, these being responsible for the physical and chemical properties of substances and we will use as an example the formation of liquid crystals commonly used in television sets, computers, calculators, among others which will allow us to understand the operation of this equipment.
INTRODUCTION
Human activity is its nature, is immersed is large amounts of substance that mostly have to make gases. However, little do we familiarize ourselves with this type of compound, since our daily use is governed by those substances that are in liquid or solid state.
On a daily basis we can observe the use of substances such as water for different tasks in our homes ranging from consumption to personal hygiene. Similarly, the use of rigid substances is common for us, an example of this, are the materials that make up our shelves in the kitchen or that are part of our car or that are present in the construction and erection of the walls in our homes.
In this sense, through the publication we will address what refers to the chemical behavior that presents this type of substance and how the forces of attraction and repulsion present in them, affect their use and produce changes related to the properties they should exhibit.
Therefore, we will make a tour of the forces of attraction involved in solids and liquids, these are the one that allows the rigidity or fluidity of substances, likewise we will address in a practical or technological way, the use of liquid crystals in our daily lives.
INTERMOLECULAR FORCES AND THEIR INTERACTION IN CHEMICAL SUBSTANCES
In this section of the subject, we will focus on the study of the type of force that can present the chemical substances and which allow them to present one or more states of matter, understood as solid, liquid and gas.
If we make a brief descriptive tour at the molecular level of the liquid substances, we can express that the molecules are bound together but with freedom of movement, which is why liquids have the ability to take the form of the container that contains it, likewise because they are fluids can spill or spread in a given space.

Fig. 2. Representation of liquid substances. Author: Aline Ponce
On the other hand, in solid substances the molecules present a marked rigidity, which allows them to present a hardness and therefore have a defined shape, a phenomenon attributed to the little space between the molecules of the substance. Undoubtedly, the molecular organization of the substances is different, in function of it seems to us opportune to raise the following question:
Why are molecules organized differently in chemical substances?
To answer this question it is necessary to address the types of interactions that can occur in a chemical compound, among which are the dipole-dipole, ion-dipole and hydrogen bridge interactions, these interactions being understood as intermolecular forces that generate an attraction between the molecules of chemical compounds.
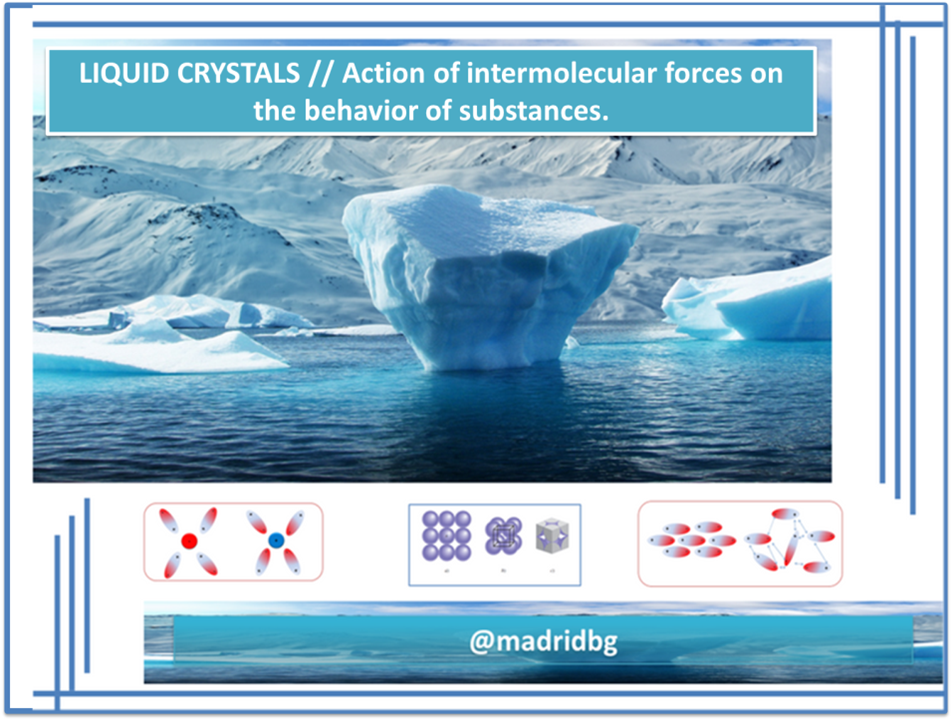
Consequently and referring to dipole-dipole interactions, we can express that it is a type of intermolecular force that occurs between polar molecules, i.e. those that have the ability to mobilize their electrons and generate a dipole moment in the molecule, so its origin tends to make electrostatic. [1]
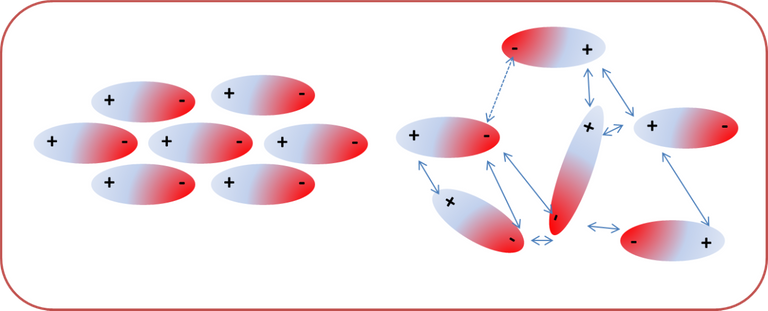
Fig. 3. Representation of the dipole-dipole interaction Author: @madridbg, through Power Point 2010.
If we analyze the organization of polar molecules presented in the previous image, we can observe that the molecules are arranged according to the dipole moment, that is, according to the movement presented by the electronic charge, where the negative charges attract the positive ones.
On the other hand, ion-dipole interactions, occur as a consequence of an attraction between molecules and an ion, which can be a cation or an anion, the intensity depending on the size of the ion and the charge of the molecule. [3]
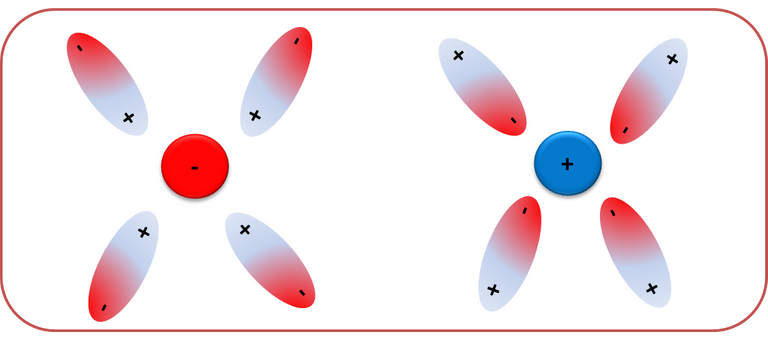
Fig. 4. Representation of the ion-dipole interaction Author: @madridbg, through Power Point 2010.
Finally, hydrogen bridges, are a type of interaction that tends to exert attraction between molecules and occurs between the hydrogen atoms of polar molecules and electronegative atoms such as nitrogen, oxygen or fluorine. The intensity of the hydrogen bridges depends on the free electrons of the electronegative atoms and the hydrogen nucleus. [1]
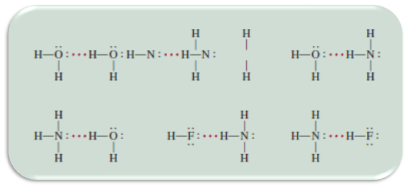
Fig. 5. Representation of the interaction by hydrogen bridges. Author: @madridbg, through Power Point 2010.
PACKAGING PROCESS OF SOLIDS AND LIQUIDS
In this section we will focus our attention on describing the process of molecular organization that occurs in solids and liquids, a process that is decisive for the form that these substances present. In the case of liquids, as mentioned in previous sections, the organization is not so rigid and there is mobility within the molecule.
In order to instruct the reader we will use as an example one of the most important substances for life such as water, the same as a liquid substance has the property of generating intermolecular forces in all directions within the liquid which generates a tension on the surface of the liquid, which is known as surface tension.

Fig. 6. Surface tension in liquids. Author: Ulrike Leone
Although there are many compounds that have the ability to form hydrogen bridges, the one that best exhibits this interaction is the water molecule, since the oxygen in the molecules makes available its electrons to generate this bridge between the molecules of the vital liquid. Thus, a three-dimensional network is generated where each oxygen atom forms a tetrahedron with four hydrogen atoms.
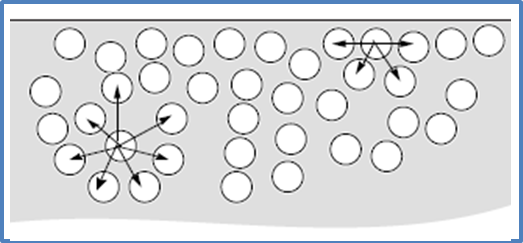
Fig. 7. Interaction in water molecules. Author: @madridbg, through Power Point 2010. Adapted from Chang, (2010).
For their part, solid substances are more complex in terms of molecule organization and can generate two types of organization. On the one hand those that adopt a specific and regular organization which are called crystalline solids and others where the arrangement is disordered and are known as amorphous solids.
In this type of substances, the molecules are organized and generate compact structures that only present a vibrational state between them. This allows them to exhibit extreme hardness and strength.
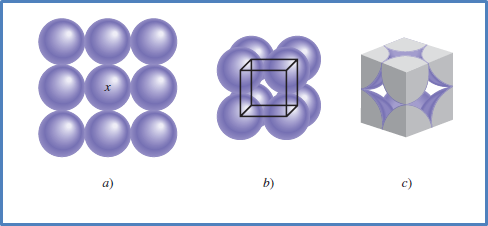
Fig. 8. Representation of molecular organization in solids. Author: Chang, (2010).
FORMATION OF LIQUID CRYSTALS
In the previous sections we studied the molecular behavior of solids and liquids and were able to establish a marked difference in the arrangement of molecules in these substances, where solids demonstrate a highly organized or ordered state, while liquids present a regular or random organization.
However, there is a type of substance that does not comply with this ordering and whose behavior is different from that studied so far. Consequently, there arise substances that present a paracrystalline state which have properties of a crystal but when heated generate a milky liquid, if the temperature increases excessively this liquid becomes translucent and the substance that was initially solid now presents the properties of a liquid.
Structurally, this type of substance has an elongated arrangement and the molecules are rod-shaped, which is why they are often called thermotropic liquids and are formed when the solid is heated.
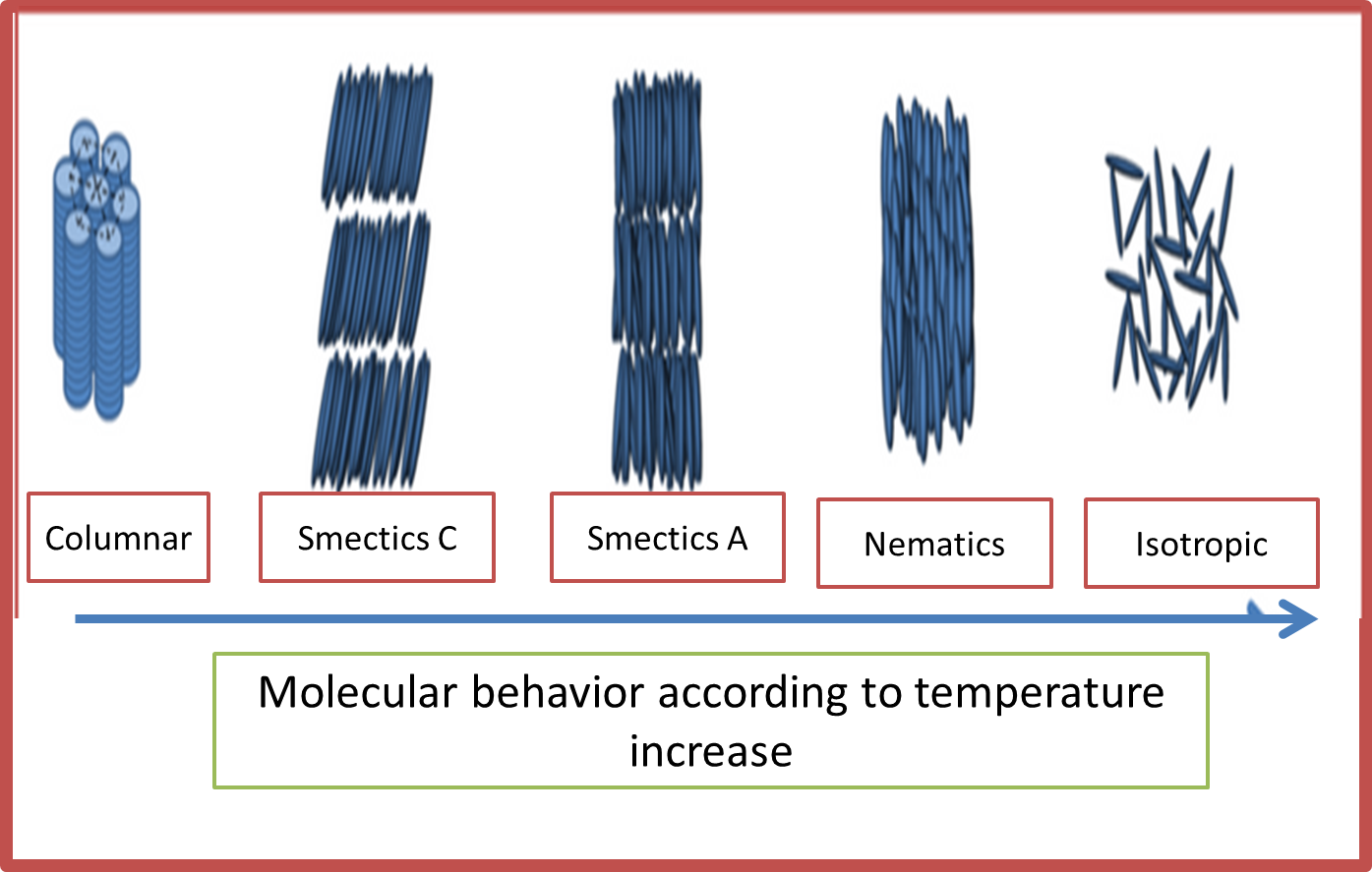
Fig. 9. Structural representation of liquid crystals. Author: @madridbg, via Power Point 2010.
These types of substances are of great technological, scientific and medicinal use. They are used in the screens of calculators, watches and even televisions, where through an electric field can induce an organization in the molecules that allows the passage of polarized light and consequently allows images to be generated on the surface of the same.
At the medical level they are used in the arrest of tumors because in the areas where they occur, usually there is usually an increase in temperature, due to the metabolic acceleration produced by the affected cells. In this sense, the doctor with the use of a thin layer of liquid crystal allows to detect the presence of the cellular problem since the high temperature causes a change in the coloration of the same.
CONTRIBUTIONS FROM THE TOPIC
Through the subject we were able to approach the structural formation of solids and liquids, as well as the behavior of these substances according to the types of interactions that are generated internally in them. On the other hand, we were able to address the formation of liquid crystals, where we demonstrated the usefulness of this type of substances.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Chávez, R y Santiago, J. (1999). Liquid crystals part I: "thermotropic liquid crystals". Pontifical Catholic University of Peru. Department of Sciences, Chemistry Section. Chemistry Journal. Vol. XIII, No 2. Article: Online Access
[3] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/BGMadrid/status/1370145833639931906
#posh twitter:
https://twitter.com/BGMadrid/status/1370145833639931906?s=20
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.