CHIRALITY IN DRUGS//Chemical basis
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome back dear members of the #hive platform and especially those users who make daily life in the community with greater academic contribution associated with the area of #stemsocial science. In this opportunity, we will address in depth those conceptual sections that refer to the chirality of drugs and their function at the metabolic level.
INTRODUCTION
When talking about chirality, it is necessary to establish patterns of comparison that allow us to understand the phenomenon under study. In this sense, the best way to understand the spatial organization of organic compounds is through the behavior of our left hand on the right and vice versa. If we analyze the same, we can observe that one is the non-overlapping mirror image of the other, that is, one image is the reflection of the other as if you were looking at it through a mirror, a behavior known as chirality.
This behavior also occurs in organic compounds where the spatial arrangement adopted by the molecules will influence the biological or pharmacological activity of the substance. Therefore, the pharmaco-dynamic and pharmaco-kinetic behavior of organic compounds associated with drugs is influenced by their chiral characteristics.
Knowing that nowadays, most of the drugs that come on the market present an optical behavior against polarized light (they are chiral) and where their function is determined by the spin that the molecule under study may present. Thus, in this publication, we will be addressing the chemical basis and the behavior assumed by enantiomers, diastereoisomers, racemic mixtures, among others, present in drugs.
CARBON STRUCTURE VERSUS OPTICAL ACTIVITY
Chemically, the carbon atom is considered a tetrahedral element, which gives it the ability to form bonds with itself and with other elements, so it is present in a large number of compounds that we know and is part of all organic matter on the planet, hence its importance and attention by organic chemistry.
Similarly, it is an element that has the ability to form four simultaneous bonds with radicals similar or different from each other. At the level of optical isomerism, when carbon binds to four different substituents without a plane of symmetry in the molecule, it results in the formation of enantiomers whose compounds are of practical interest at the level of the pharmacological industry and which are conceived as substances that have a non-superimposable mirror image, a concept that we addressed in the previous section.
Fig 2. Chirality in carbon atoms. Author: @madridbg, via Power Point 2010.
At this point it is necessary to remember that optical activity is limited by two conditions that allow chirality within molecules. The first one refers to the presence of a chiral atom (atom with four different substituents) and the second one, that there is no plane of symmetry in the molecule as shown in the following image.
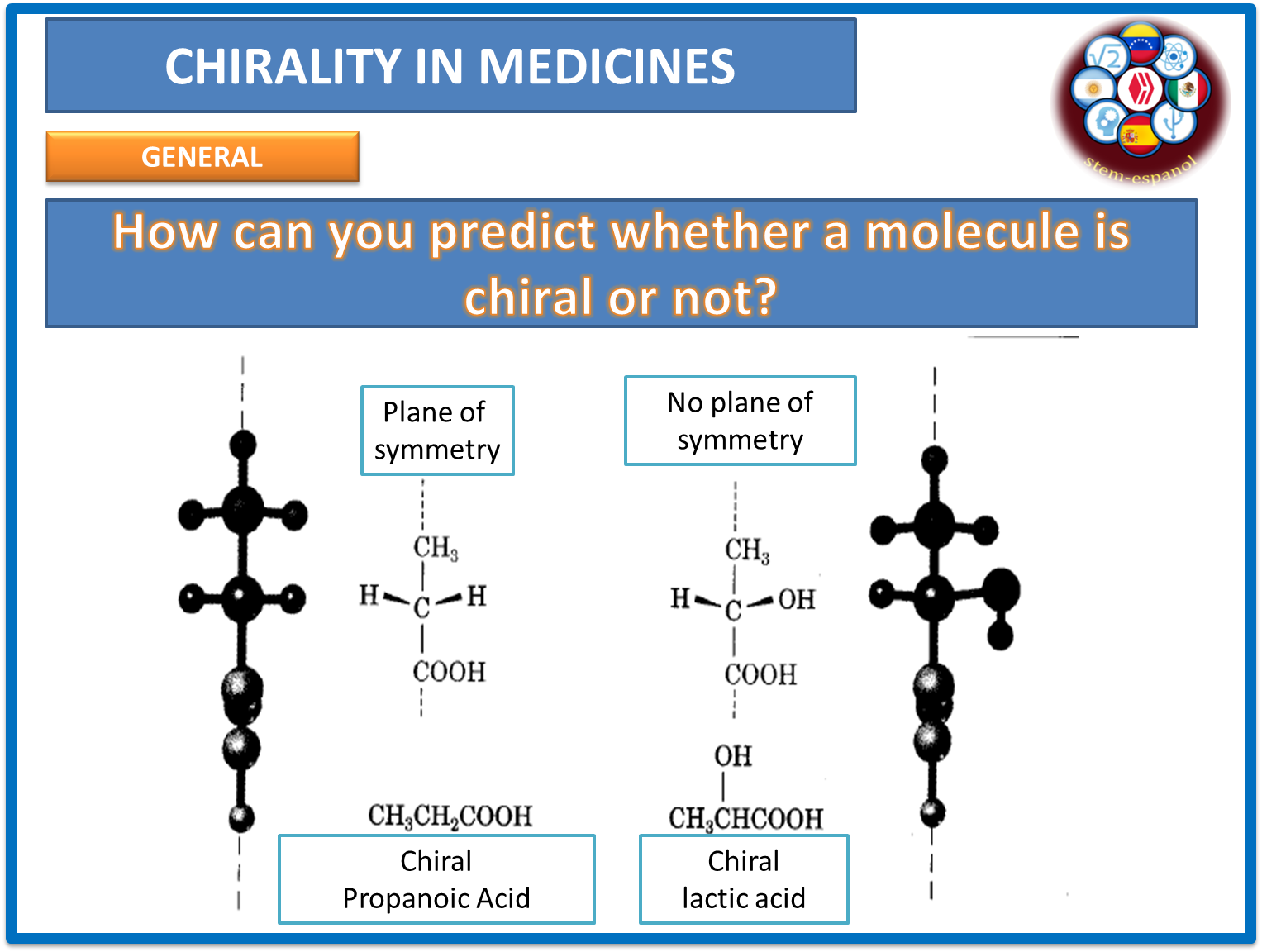
Fig. 3. Ways to predict chirality in molecules. Author: @madridbg, via Power Point 2010.
Unlike enantiomers which are formed with the presence of a chiral atom, diatheroisomers, are the result of a molecule with two or more chiral centers which gives rise to different compounds that have the same type and number of atoms, the same physical and chemical properties but differ in their biological and pharmacological behavior.
Similarly, in those molecules where there are 2 or more chiral centers may appear substances called meso compound and are produced because of an internal symmetry in the molecule, which when rotated 180 ° ends up forming the same compound and the expected diatheroisomers are not generated.
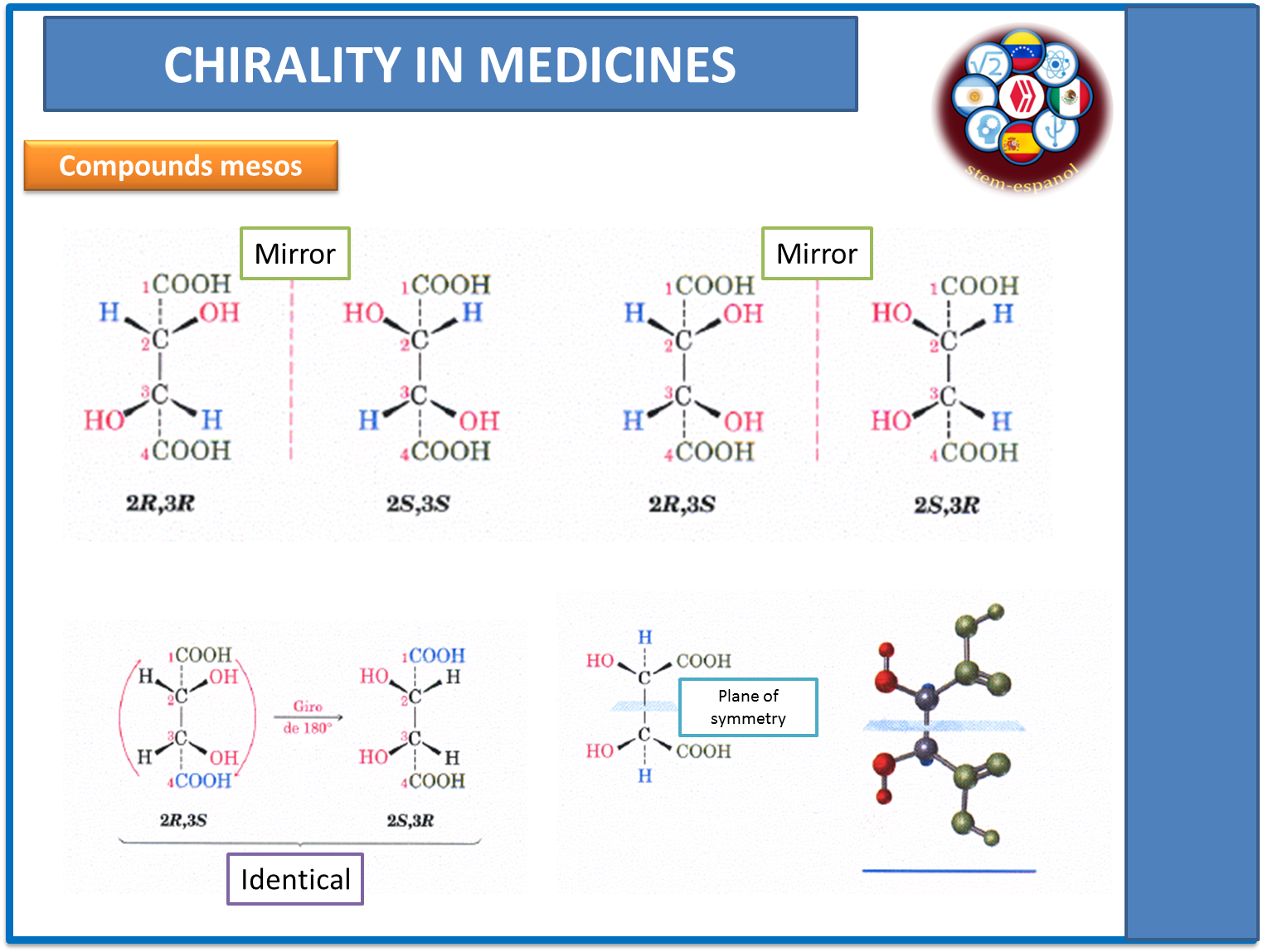
Fig. 4. Internal symmetry in meso compounds. Author: @madridbg, through Power Point 2010.
Racemic mixtures, another type of substance generated by chirality, refer to the fact that in the same molecule there are both the enantiomers and their mirror image in concentrations of 50-50, so that the optical activity cancels each other out. This type of molecule, together with the enantiomers, are the most studied by the pharmacological industry due to their different pharmaco-kinetic and pharmaco-dynamic behavior.
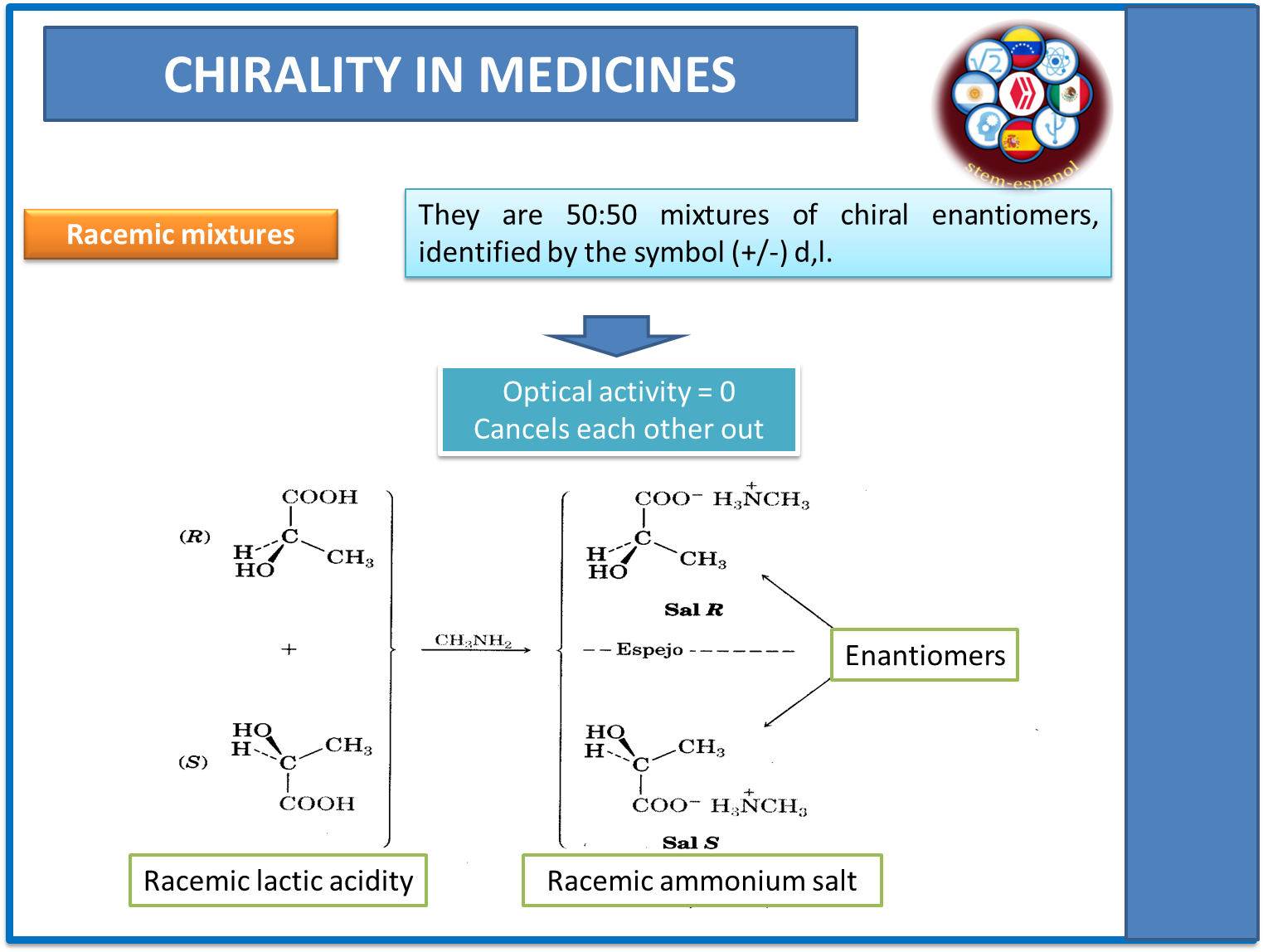
Fig. 5. Racemic mixtures and optical activity. Author: @madridbg, through Power Point 2010.
THE POLARIMETER IN THE DETECTION OF OPTICAL ACTIVITY
In previous publications, we dealt with electromagnetic radiation. In this regard, it is appropriate to recall that ordinary light travels in different directions in such a way that it becomes polarized when it manages to concentrate in a single plane, a function that fulfills the polarimeter. Once the light is polarized, it is matched with the sample of the substance under study to determine the twist it causes in the polarized light.
Through the behavior of the light, it can be established whether the substances are dextrorotatory (turns the plane of the light to the right) or levorotatory (turns the plane of the light to the left), giving a glimpse of the presence of optical activity in the chemical molecule under study.
This behavior was pointed out by Jean Baptist Biot, a French scientist who studied the nature of light in a plane and who made the first observations about the behavior of certain chemical molecules in polarized light.
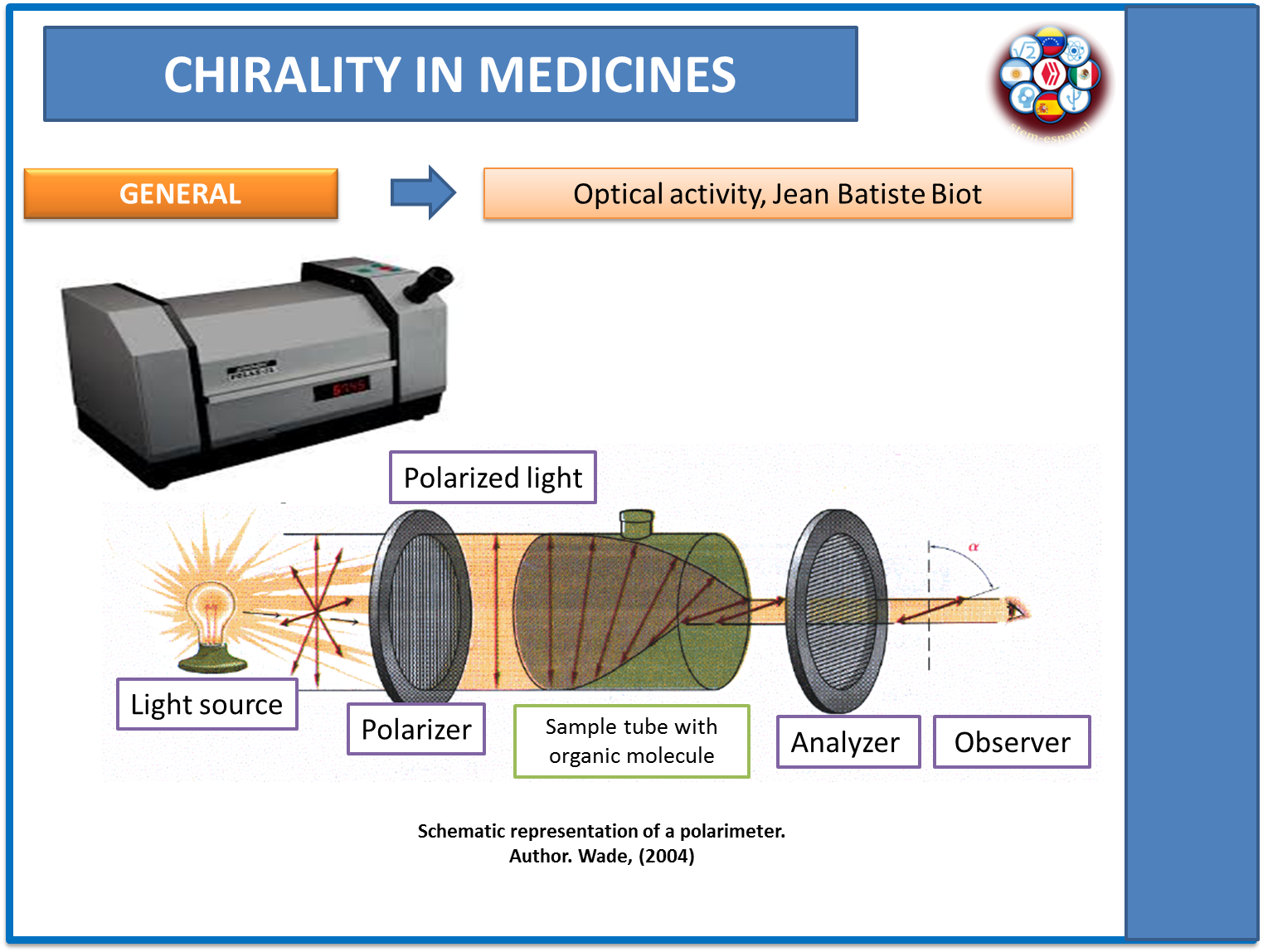
Fig. 6. Polarimeter operation. Author: @madridbg, using Power Point 2010. Using Wade's contributions. (2011).
SPATIAL CONFIGURATION OF ATOMS AND CHIRALITY
As mentioned in the previous section, the polarimeter allows us to determine whether the molecule is dextrorotatory or levorotatory, but we cannot determine through this, the configuration or spatial arrangement adopted by the different atoms that are present in the chemical molecule. Therefore, following the investigations carried out by Cahn-Ingold-Prelog, a sequence of steps or rules can be established to predict the spatial behavior of substances.
These are summarized below:
Step 1: Use as a reference standard the atomic number of atoms present in each substituent attached to the chiral atom and establish hierarchy according to the largest molecule.
Step 2: If it is not possible to establish an order between the molecules using the first atom, move on to the next one until the first difference is achieved.
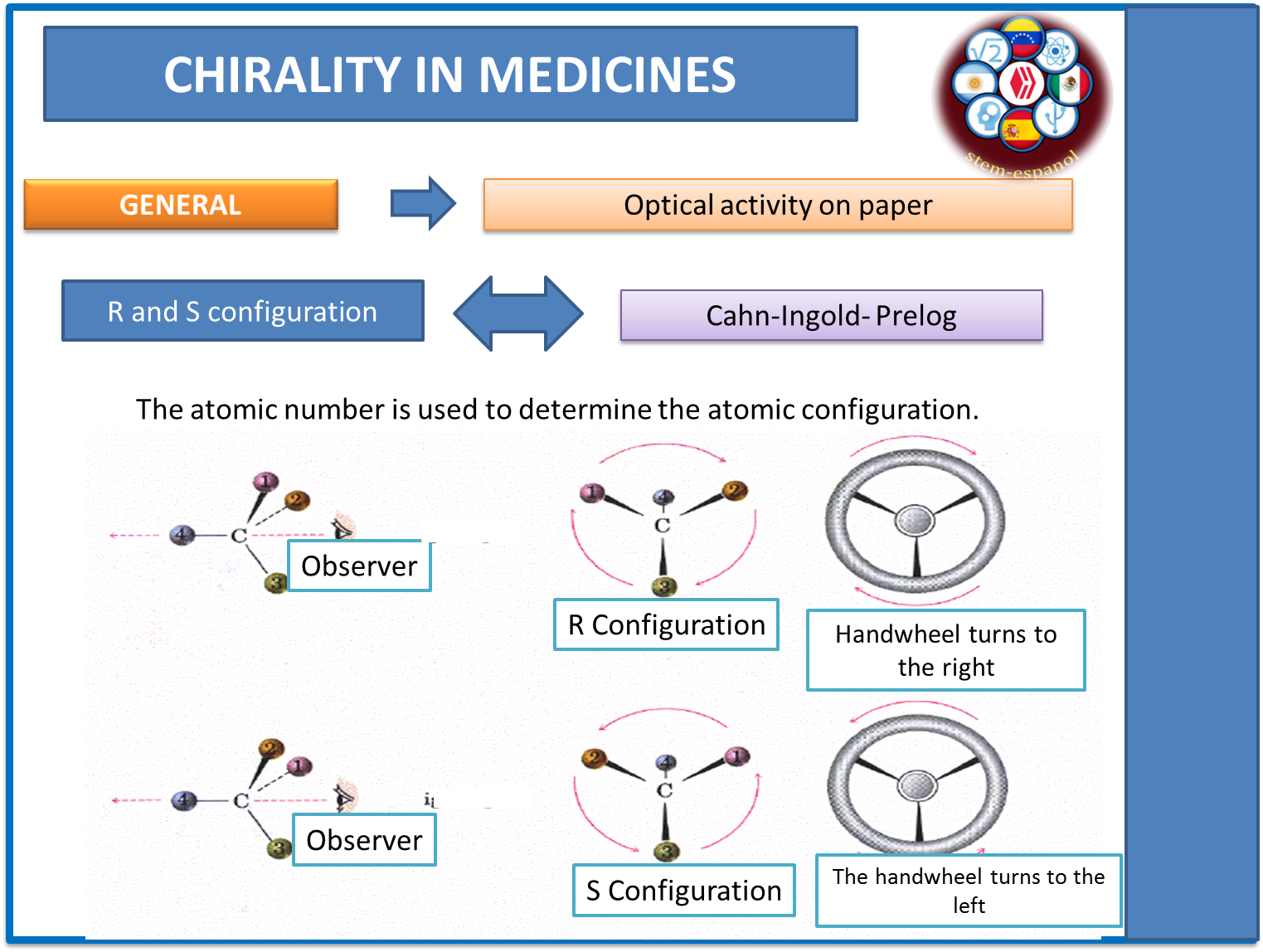
Fig. 7. R and S configuration and optical activity. Author: @madridbg, via Power Point 2010. Using input from Wade. (2011).
Once the priorities of each substituent are marked, a line is drawn in the trajectory of the substituents, which will not allow to establish the spatial configuration of the substance, under the configuration (R and S) according to the twist that is presented.
R = the spin occurs to the right or clockwise.
S= the turn occurs to the left or counterclockwise.
CHIRALITY IN DRUGS: DUAL BEHAVIOR OF CHEMICALS
At the industrial level, the active ingredients used in the manufacture of drugs come from nature and others by synthesis or chemical modifications at the laboratory level. The latter are the most problematic at the pharmacological level because they can be presented in the form of racemic mixtures and not as pure enantiomers.
Therefore, in this section we will describe some disadvantages and drawbacks of the uses of racemic drugs instead of pure enantiomers. Recall that a racemic mixture is a 50-50 combination of the enantiomer and its mirror image, i.e. 50% is levorotatory and the other 50% is dextrorotatory, so despite having the same properties their biological and pharmacological behaviors are different since the spatial organization of the atoms is different.
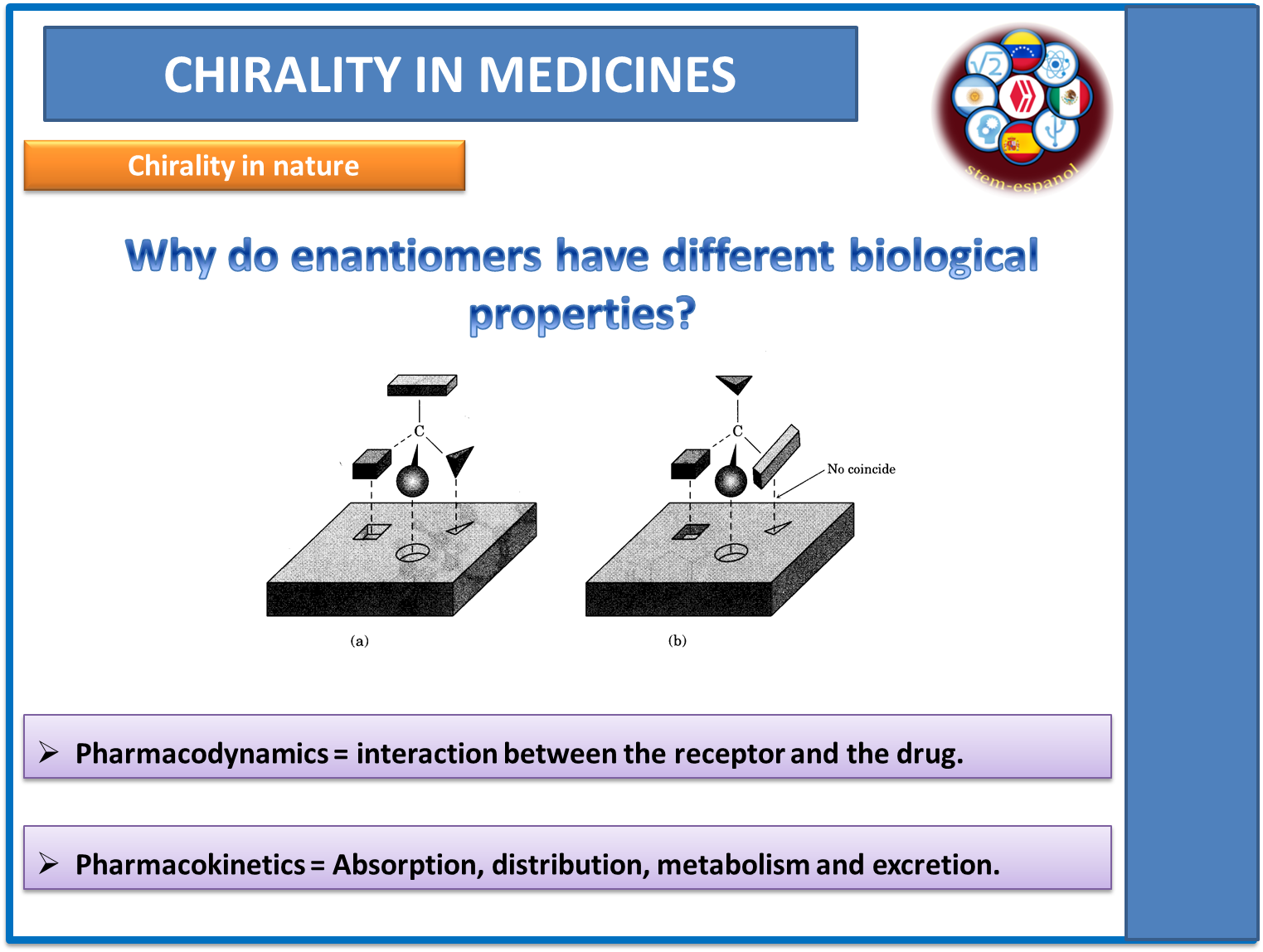
Fig. 8. Specificity of chiral molecules. Author: @madridbg, via Power Point 2010. Using contributions from Chang. (2010)
The reason for their behavior is due to the interaction between the drug and the active sites of the substrate, which as we can observe in the image above, these substances present a specificity like a lock with its key and are governed by the same principles of enzymatic functioning, so the pharmaco-dynamics and pharmaco-kinetics become more complex in racemic mixtures since each enantiomer must be studied separately.
An unfortunate example of this behavior is the drug thalidomide, a drug in the form of a racemic mixture, which was prescribed as a sedative and as a nausea reliever in pregnant women, to some extent the drug under its (R) configuration fulfilled the stipulated pharmacological purpose. To the surprise of many, the (S) configuration caused havoc in the fetus of the women who consumed it. Marked teratogenic effects were detected in the children at birth, presenting malformations in the upper and lower limbs.

Fig. 9. Collateral damage from thalidomide. Author: Niko von Glasow
From there, lies the importance of making this information known and where, at the pharmacological level, daily studies are carried out to generate drugs based on pure enantiomers, since these are more efficient and effective at the time of treating a pathology.
CONTRIBUTIONS OF THE SUBJECT
The contribution of the topic will be approached from two points of view, the first refers to my participation in the discussion, which allowed me to transmit my knowledge in the area of chemistry with people from different academic context where it was possible to demonstrate how refreshing and nutritious this type of activity is. Secondly, through the publication it was possible to reinforce what concerns chirality and to evaluate the importance of generating the necessary chemical studies to medicines before putting them on the market, so that no more cases like that of thalidomide occur.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Juaristi Eusebio. (2005). Izquierda y derecha en química: la quiralidad. Ciencia. Article: Online Access
[3] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] Túnez Fiñana Isaac, Galván Cejudo Aurora, Fernández Reyes Emilio. pH and buffers: Physiological buffers. Department of Biochemistry and Molecular Biology, School of Medicine. Córdoba.Artículo: Acceso Online
[6] Vaquero Juan José. Metodologías químicas en el descubrimiento de nuevos fármacos. Universidades de Alcalá, Complutense y S. Pablo-CEU Doctorado Interuniversitario QUIMICA MEDICA. Article: Online Access
[7] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/BGMadrid/status/1374503095263105039
#posh twitter:
https://twitter.com/BGMadrid/status/1374503095263105039?s=20
Congratulations @madridbg! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
Your next target is to reach 900 replies.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz:
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.