APPLIED CHEMISTRY// Nyos the Killer Lake
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of the #hive platform who are passionate about content from a scientific perspective. Continuing with the series CHEMISTRY IN CONTEXT, today we will be talking about "NYOS THE KILLING LAKE", an interesting topic that will allow you to know, a natural event with a lot of chemistry.
INTRODUCTION
Planet Earth is made up of 71% water, of which 3% is in the form of ice. Similarly, the human body is formed mostly (66%) by this wonderful liquid. Therefore, it is not a matter of surprise that many of the chemical processes that occur on the planet take place in watery environments.
These processes generally bring about thermodynamic changes that allow the transformation or not of the substances involved. They can occur in the three states of aggregation of matter, understood as solid, liquid and gaseous, where atoms and molecules interact through intermolecular forces that allow maintaining the union of them.
In this particular, we will focus on studying the transformations and interactions of matter. Making a tour of the units of concentration used and the effect of pressure and temperature on some systems in solution.
DISSOLUTIONS: A MOLECULAR APPROACH
As explained in the previous topic, the understanding of chemical processes begins by observing our surroundings and questioning how and why things happen. In this sense, if we look around us we will see that most of the materials in our daily lives are combined or part of a mixture. To cite some examples we can mention:
1. Air, a gaseous mixture of oxygen and nitrogen.
2. Gasoline, a liquid mixture of different hydrocarbons.
3. The rocks, solid mixture of different minerals.
Under these criteria, in this section we will study a particular type of mixture called DISSOLUTIONS, the same can be defined as a homogeneous mixture formed by a solute and a solvent. When the interaction solute (A) - solvent (B) occurs, it implies that there is a dispersion of A in B respectively, the particles of the solute occupy the spaces that were occupied by the solvent.
Referring to the solute, we can express that it is the substance that is found in smaller quantity and therefore, it dissolves. As for the solvent, substance that is found in greater quantity and therefore dissolves.
In order to understand in detail what happens, let's review the following sequence:
Stage 1: Separation of the molecules from the solvent
Stage 2: Separation of molecules from the solute
Stage 3: It occurs as a result of the ruptures of the intermolecular attraction forces in stage 1 and 2, and the molecules are already prepared for the union, it is there, where the mixture occurs.
According to the amount of dispersion of A into B that exists, the solutions can be classified in Chang's opinion. (2010) in saturated, is the one that has the maximum capacity of solute that the solvent can dissolve.
Unsaturated, the amount of solute is small compared to the amount of solvent present. On the other hand, the supersaturated solutions , are the most unstable and have a greater quantity of solute than that present in the saturated solutions.
Another aspect that we must mention, are the types of relations that can be presented in a solution. See in detail in table 1.
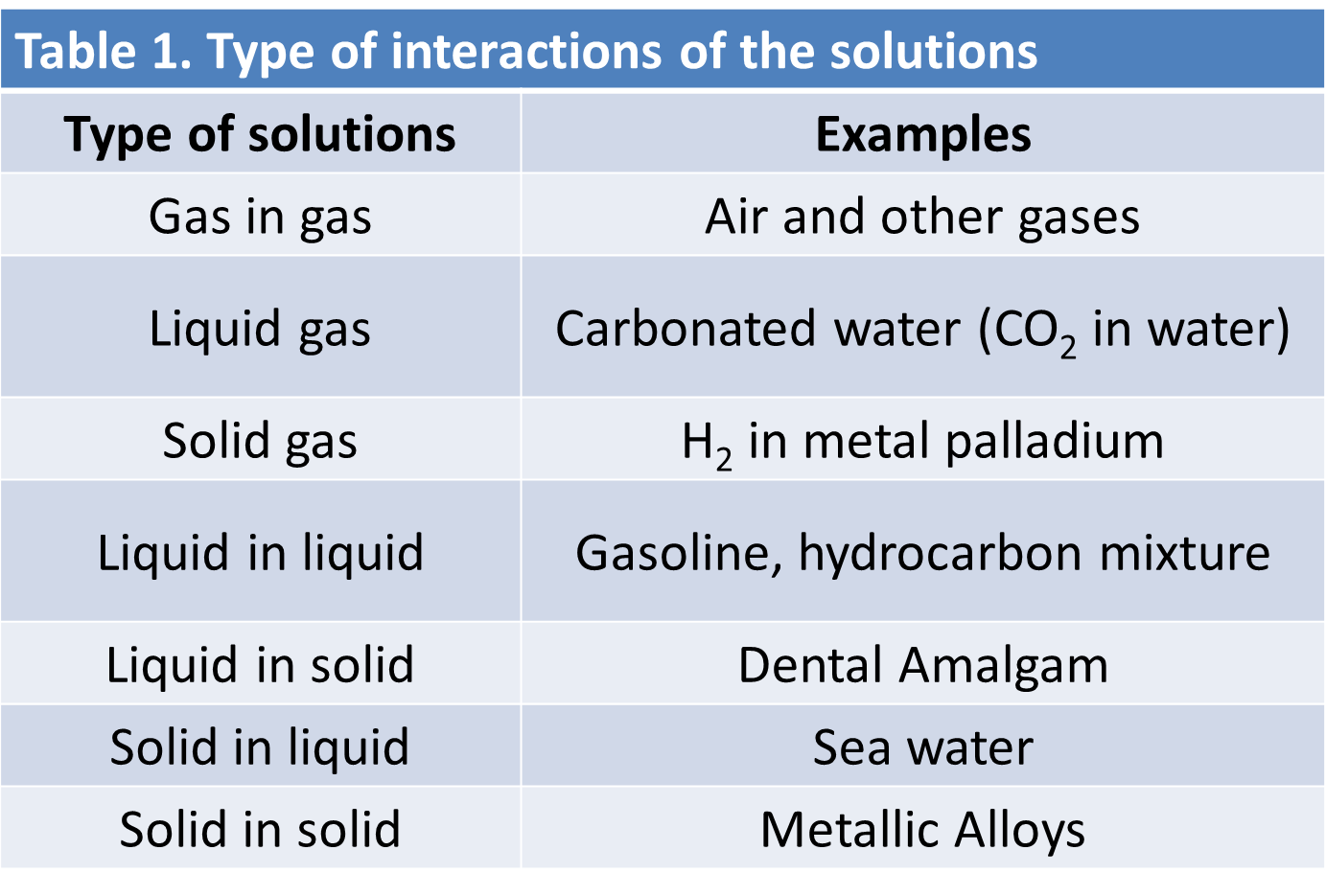
Table 1. Type of interactions of the solutions. Author: @madridbg, through Power Point 2010., Adapted from: Chang, (2010)
CONCENTRATION OF THE SOLUTIONS
In order to achieve the task posed in this subject, it is necessary to understand that the solutions are formed by a solute (A) and a solvent (B), where A is dissolved in B, from this we can say that the solution is concentrated or diluted. However, in the scientific or experimental work the important thing is to know exactly the concentrations with which one is working.
In this sense, there are several ways to determine such concentration, they can be classified into physical units and chemical units, see image 1 and 2. In reference to the physical units, we will mention the percentage units such as the percentage in mass and volume.
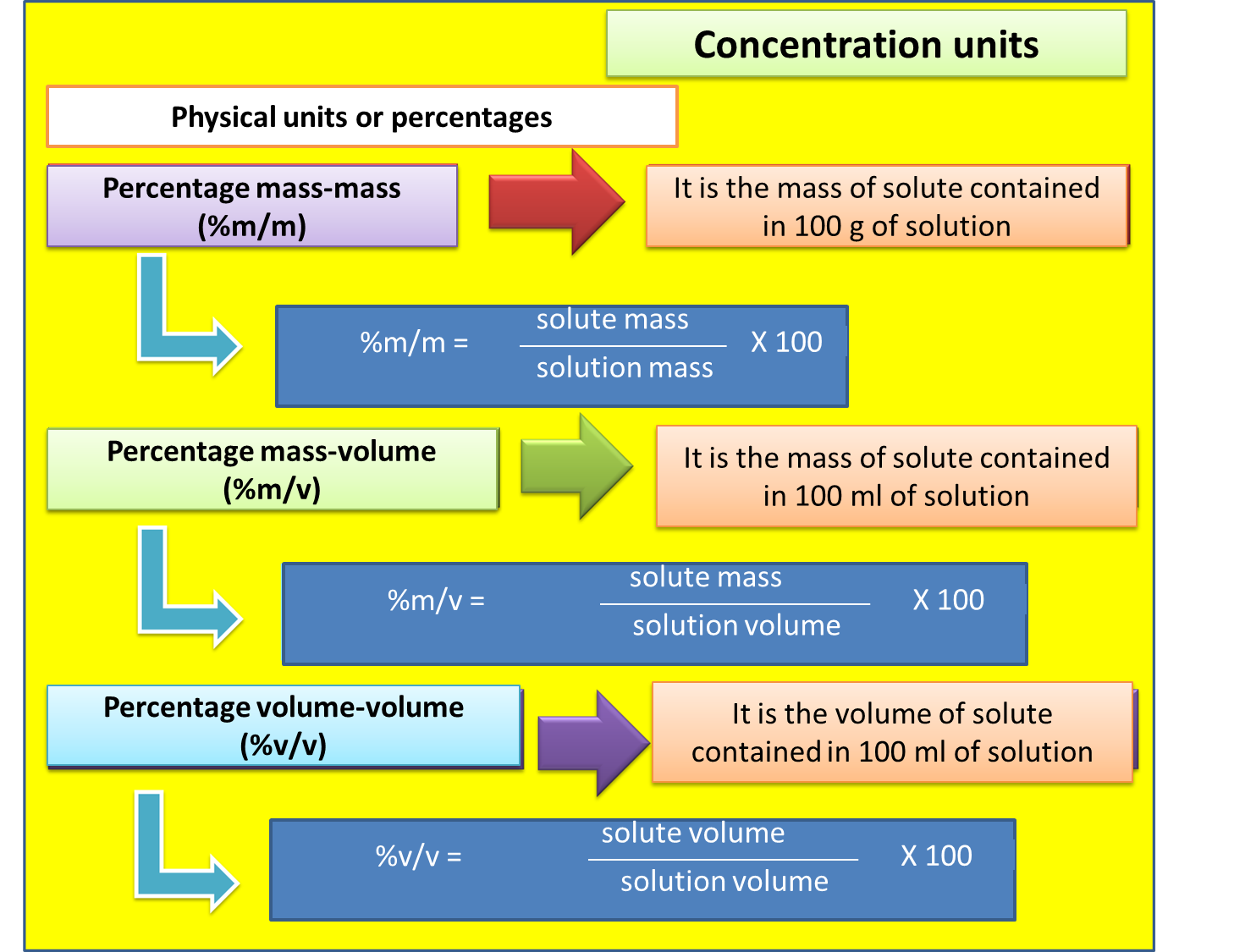
Fig. 2. Physical units of the solutions.. Author: @madridbg, through Power Point 2010.
As for the chemical units, we will mention what is related to normality and molarity, focusing in depth on the latter, in order to understand the phenomenon manifested in the title of this topic.
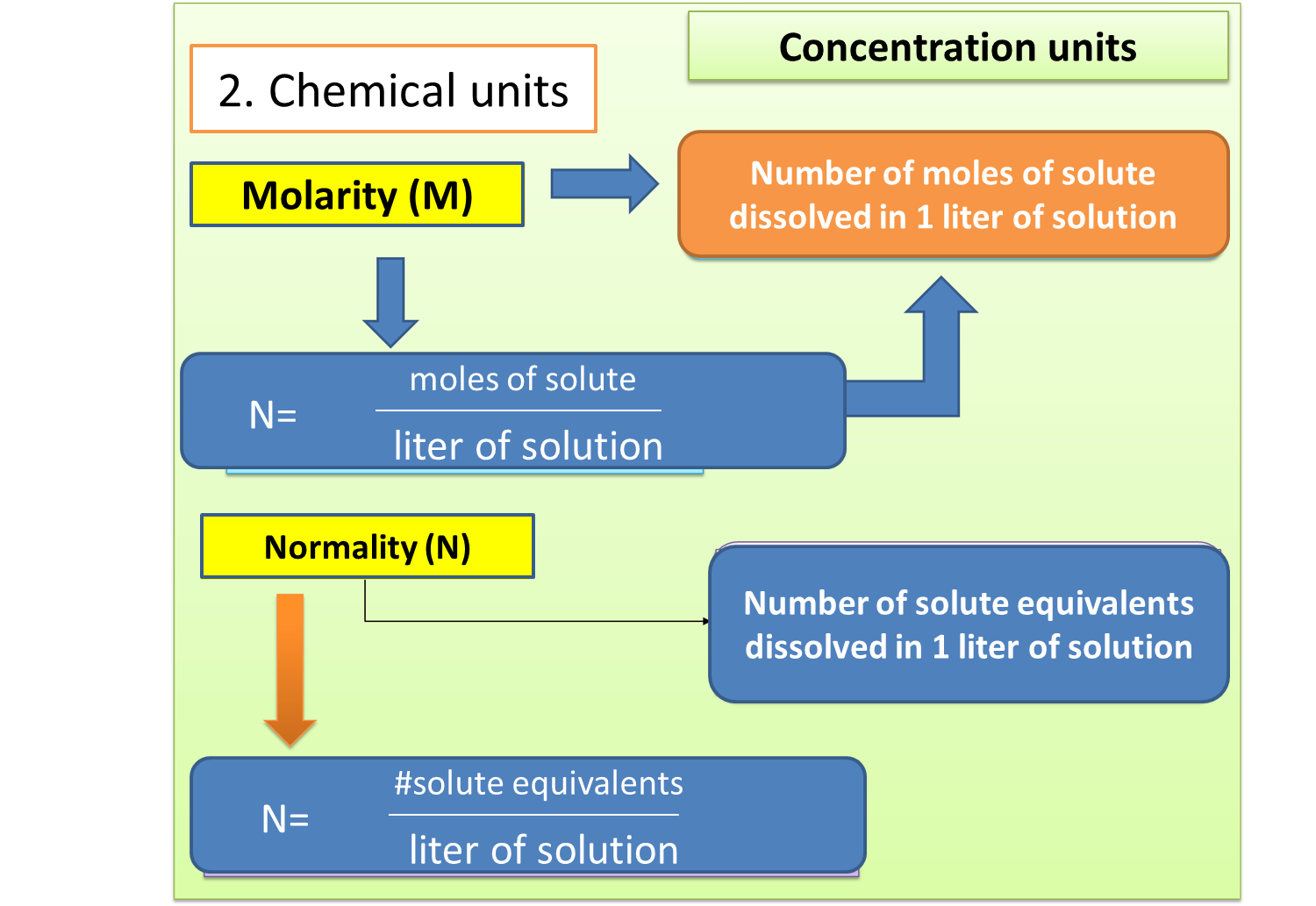
Fig. 3. Chemical units of the solutions. Author: @madridbg, through Power Point 2010.
In order to instruct the reader in the calculation of molarity we will do it with the following practical exercise:
5 liters of a saline solution are prepared with a concentration of 0.100 Molar. Determine the amount of salt or sodium hydroxide (NaOH) needed for this task. Molecular weight of NaOH is 40g/mol.
In order to respond to this approach we will follow the following sequence.
Step 1: Read the exercise carefully.
Step 2: Extract the data provided by the exercise.
Step 3: Find the way or form that allows us to reach the solution.
Step 4: Perform the necessary mathematical operations.
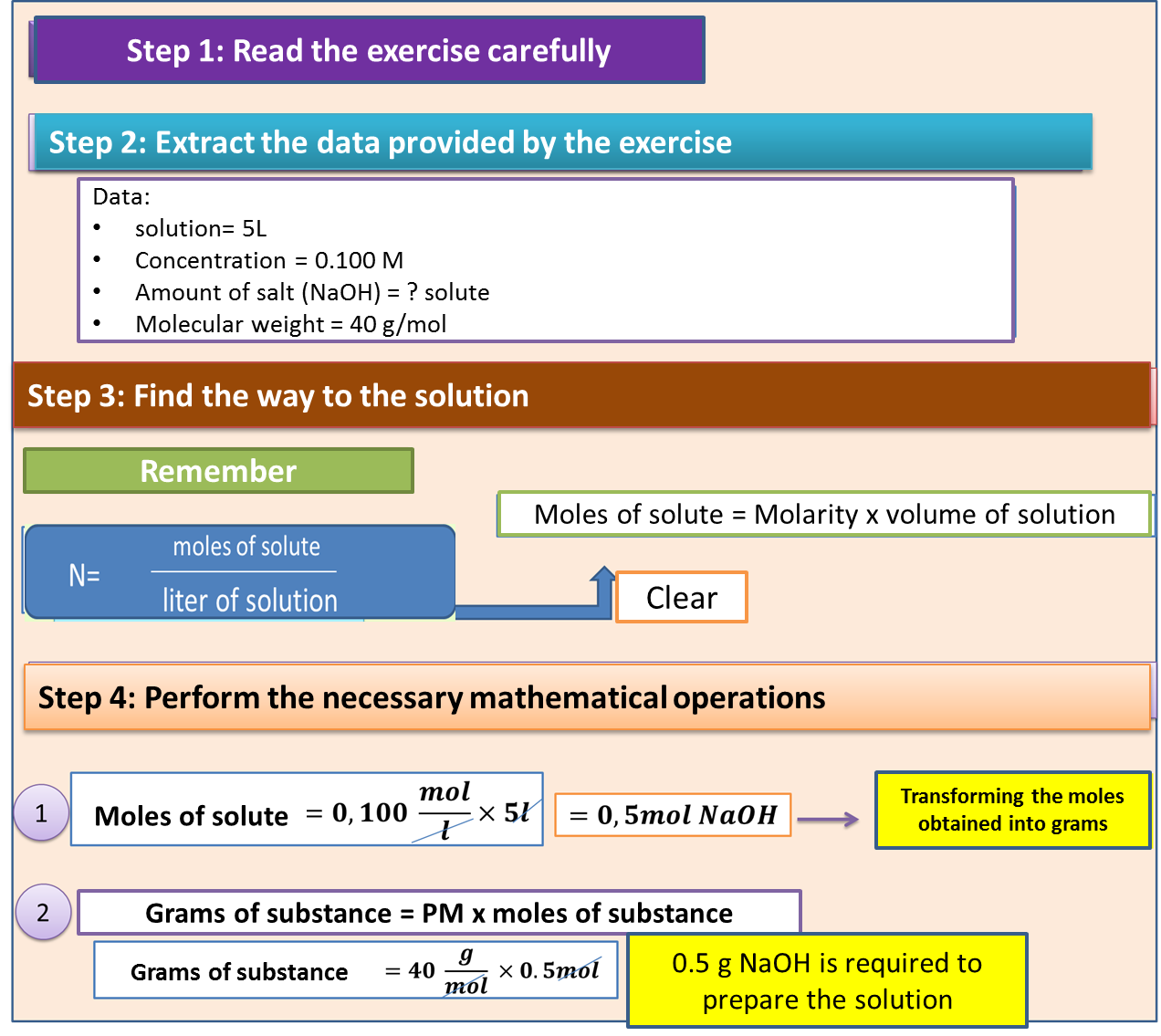
Fig. 4. Procedure to calculate molarity. Author: @madridbg, through Power Point 2010.

Fig. 5. Sequence to solve the exercises. Author: @madridbg, using Microsoft GIF animator.
THE KILLER LAKE: EFFECT OF TEMPERATURE AND PRESSURE ON THE SOLUBILITY
On August 21, 1986 in Cameroon, a country on the African coast, a natural incident arose that devastated the lives of more than 1700 people. Lake Nyos suddenly expelled a dense layer of smoke responsible for so many deaths. Today, thanks to chemistry, it is possible to predict the cause of this misfortune.
To guide the reader on what happened, it is necessary to approach the concept of solubility of the substances, understood as the capacity that has the solvent to dilute certain solute to a specific temperature, the same one is measured in (grams of solute dissolved in 100 ml of solvent). See image 6.
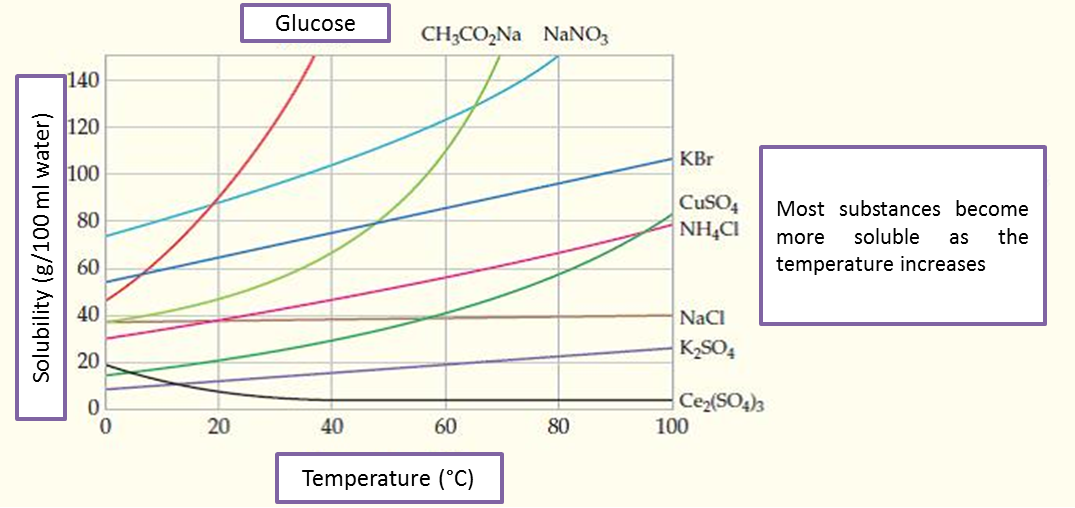
Fig. 6. Solubility of some substances. Author: Mcmurry y Fay (2008).
The solubilities affected to the different states of aggregation of the matter (solid, liquid and gaseous) and is influenced by pressure and temperature. In this particular, we will focus on studying the solubility of gases and how it is affected by the variables mentioned above.
Referring to the influence of temperature, it can be predicted that solubility decreases as the temperature increases. This phenomenon can be observed in carbonated beverages, which bubble continuously as they are heated, becoming lighter, because they have lost carbon dioxide (CO2) dissolved.
In reference to the pressure, we can mention the postulate of Henry's law, which establishes that the solubility of a gas in a liquid, is proportional to the pressure of the gas on dissolution. In other words, by increasing the pressure of the gas, the solubility of the liquid increases. Quantitatively the law is predicted according to the amount of molecular collisions of the gas against the surface of the liquid and that they are trapped in the condensed phase. [1]
Extrapolating the previous topics with the Lake Nyos incident, it can be explained in Molerio's opinion (2014), that the cause of this event is due to the dense dissolution formed in the depths of the lake, which contained large amounts of minerals and dissolved gases, among which were found in CO2.
Due to the difference in pressure between the surface water and the water at the bottom of the lake, the concentration of CO2 gradually accumulated until it became a potential disaster, according to Henry's Law. When the bottom water emerged, dissolved carbon dioxide separated from the solution and became a silent killer, as it was heavier than oxygen and traveled close to the ground, suffocating its victims.
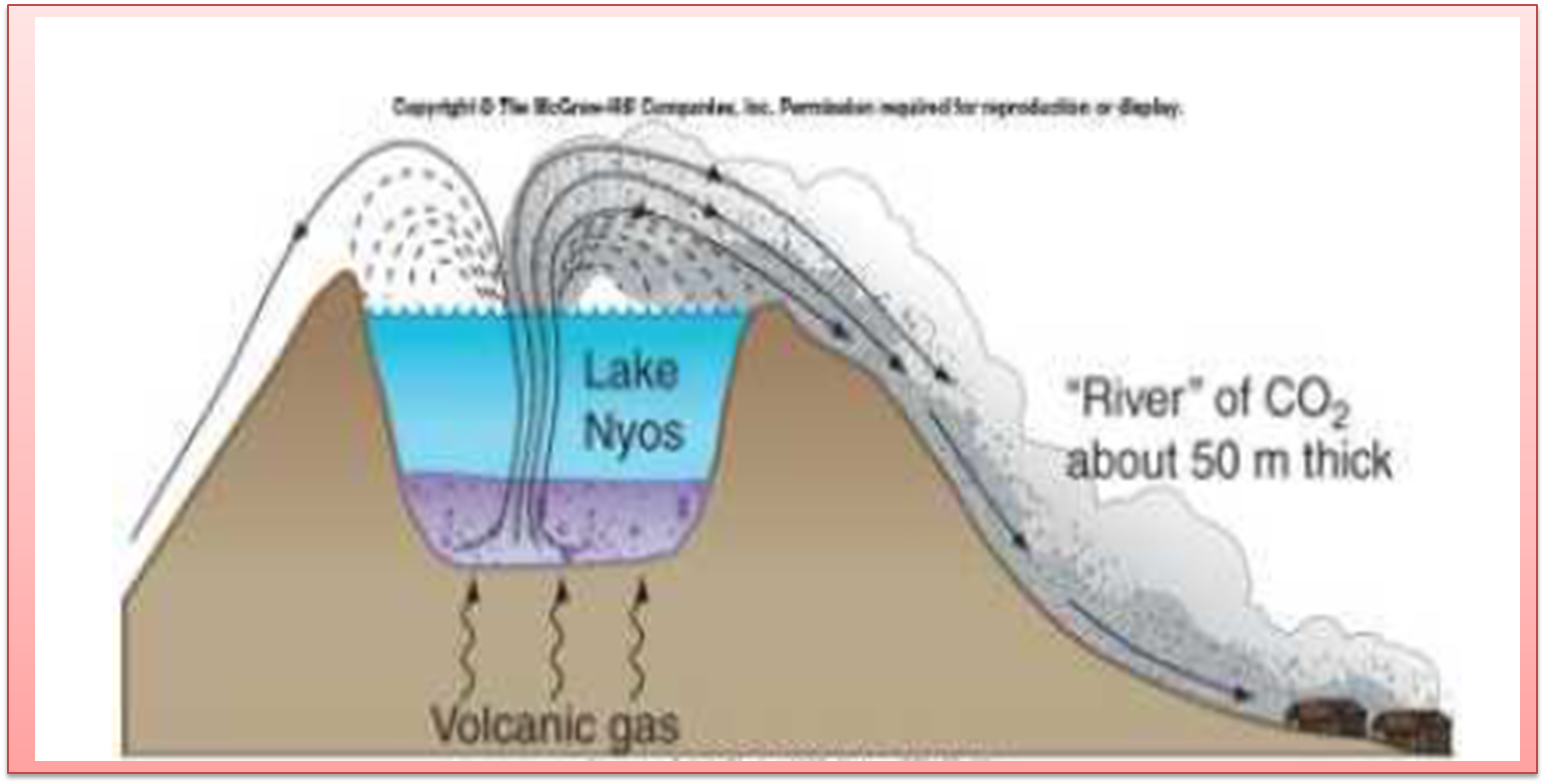
Fig. 7. CO2 leaching and gas eruption in Lake Nyos. Author: Molerio (2014).
CONTRIBUTIONS OF THE THEME
Once again it is demonstrated that Chemistry as a Science allows us to understand the functioning of the simple and complex things on our planet. Through its laws and topics we can give answers to the different phenomena of our daily life, just by questioning and contextualizing what happens.
In this sense, the theme allowed the formation of interrelations between the academic content and real experiences. In a personal way, it is a didactic reference that can be used in the different levels of academic formation.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2002) . Bioquímica. PEARSON EDUCACIÓN, S. A., Madrid, ISBN: 978-84-832-2694-0. Materia: Bioquímica, 577. ÁREA: CIENCIAS
[3] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[4] Molerio L, (2014). Volcanism in Equatorial Guinea and associated natural hazards. Inversiones GAMMA, S.A. Electronic magazine of the Environment Agency. No.26. ISSN-1683-8904. Article: Online Access
[5] Lopez, A (2013). Absorption of carbon dioxide, at high partial pressures, by aqueous solutions of alkanolamine binary mixtures. University of Jaén. Faculty of Experimental Sciences. Article: Online Access
[6] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[7] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
#posh twitter
https://twitter.com/BGMadrid/status/1340847892110700545?s=20
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Congratulations @madridbg! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Very interesting! Thanks for sharing with us :)
!discovery 30
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Delegate to us for passive income. Check our 80% fee-back Program