APPLIED CHEMISTRY// Gas laws from diving dynamics
Author: @madridbg, through Power Point 2010, using public domain images.
Greetings dear members of the #hive community, continuing with the socialization of the contents of chemistry from a practical utility, this time we will be addressing the behavior of gases according to Dalton's law and its influence on a sport like diving.
As has been constant in my publications, I share this type of content through the @stemsocial community, which does an extraordinary job in terms of contributions on topics related to the scientific area under a flexible and dynamic research structure.
INTRODUCTION
In nature we can find a great variety of substances that exhibit a behavior depending on the state of matter present and that we can classify as substances in solid, liquid and gaseous state. In this sense, we will focus on addressing the behavior of gaseous substances, since as living beings we are immersed in high concentrations of these, among which are oxygen (O2), nitrogen (N2) and others that products of the industrialization of cities have become a source of pollution for our planet, such as carbon dioxide (CO2).

Fig. 2. Gaseous molecules adapt to the container containing them. Autor: Shafin Al Asad Protic
The behavior of gases is reflected in terms of the variables pressure and temperature, which are the ones that delimit the behavior of the substance. However, for the practical suitability of these lines of research, we will focus on the approach of those gaseous substances under common atmospheric conditions, i.e., 1 atmosphere of pressure and a temperature of 25 °C.
Under the conditions already established, diatomic substances such as oxygen and nitrogen, are gases at room temperature, on the other hand, polyatomic substances such as carbon dioxide and methane are gases that exhibit liquid behavior at room temperature, however, as the temperature rises they convert to gas very easily.
As has been mentioned in other publications, gases have the ability to:
1. Take the shape of the container that contains it.
2. Being confined they can be mixed uniformly and thoroughly.
3. They are the most comprehensible of the three states of matter, due to the separation exhibited by the molecules of these substances and the few intermolecular forces they exhibit.
In this sense, through this publication we will address the behavior of gas mixtures, through Dalton's law and its implementation and effects on diving, as well as a tour of the different chemical fundamentals that apply in this sport.
GAS PRESSURE AS A FUNCTION OF ATMOSPHERIC PRESSURE
Atmospheric pressure, affects everything around us and we humans act as if this type of force did not exist, which is attributable to the adaptation we have had on it, a practical example to exemplify the presence of atmospheric pressure, A practical example to exemplify the presence of atmospheric pressure is applied when we ingest a drink through a straw, at the moment of sucking we extract the air inside the straw and the empty space generated is filled with the liquid which rises being pushed by the pressure that the atmosphere exerts on the substance.

Fig. 3. Atmospheric pressure is responsible for the liquid flowing through the straw. Autor: JapoDelMal
Gases do not escape this reality and because of the constant kinetic motion of the molecules they tend to exert a pressure on the surface of the materials with which they come into contact. Therefore, if we want to determine the pressure generated by gases we must make a correlation between the force applied per unit area so that we can conceptually state the pressure of substances.
At the atmospheric level, the pressure depends on the gravitational force of our planet, therefore it is expected that the closer we are to the ground the greater the pressure exerted by the atmosphere, product of a higher density of air molecules present, so that the atmospheric pressure is dependent on the temperature, the type of climate and the place where we are. At a practical or experimental level this type of force is measured with an instrument called barometer, which allows us to measure the pressure supported by a column of mercury at a height of 760 millimeters and 0 °C above sea level.

THE PARTIAL PRESSURES OF GASES FROM DALTON'S LAW

Fig. 4. Equipment adapted to measure atmospheric pressure. Autor: UTLA
Another aspect that we must take into account, is that the atmospheric pressure is given vertically from top to bottom, however the air being gaseous and fluid tends to exert interaction in all possible directions, product of the constant collisions that are generated between the molecules.
THE PARTIAL PRESSURES OF GASES FROM DALTON'S LAW
The air that we consume in the respiratory process, is not pure oxygen in its entirety, on the contrary it is a mixture made up of different gases, where each of these substances exert a partial pressure when in contact with an object. Based on this premise in 1801, Dalton enunciated the gas law, where he states:
"The total pressure of a mixture of gases is equal to the sum of the pressures that each gas would exert if it were alone." [3]
Consequently, measuring the independent pressure becomes a much more complex process, because the manometer only measures the total pressure of the system, in this sense we must resort to the use of mass spectrometry technique so that the signals emitted allow us to predict the quantities of substances present and consequently the mole fractions of the same. Based on these variables we apply the equation presented by Dalton and determine the independent pressure of the gases present in the mixture.
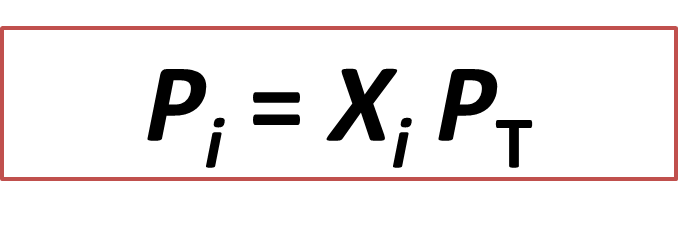
Ecuación 1: Representation of Dalton's law. Autor: @madridbg, a través de Power Point 2010, adaptado de: Chang, (2010).
If we analyze the above equation we realize, that it is related to the total pressure (Pt) of the system and to the mole fraction (Xi) of each independent substance, so that we can determine the partial pressure (Pi) of the substance we are interested in.
BEHAVIOR OF GASES AND THEIR IMPACT ON DIVING
Thanks to the laws of gases, diving has become a safe sporting activity for those people trained and experienced in the area, this due to the development of specialized equipment with certain autonomy associated with underwater breathing, which control the amount and concentration of oxygen and nitrogen necessary for a safe dive, so as to avoid fatal damage to the body resulting from an inadequate expansion of these substances in our interior.

Fig. 5. Diving as a recreational sport. Autor: PxHere
Therefore, the operation and behavior of the pressure in the depths of the sea is attributed with an increase of this variable as the depth increases, in this sense, at a depth of 33 feet is estimated a partial pressure of 1 atmosphere equivalent to that of air, if this depth doubles, the pressure also doubles, so we can establish that they are directly proportional. [1]
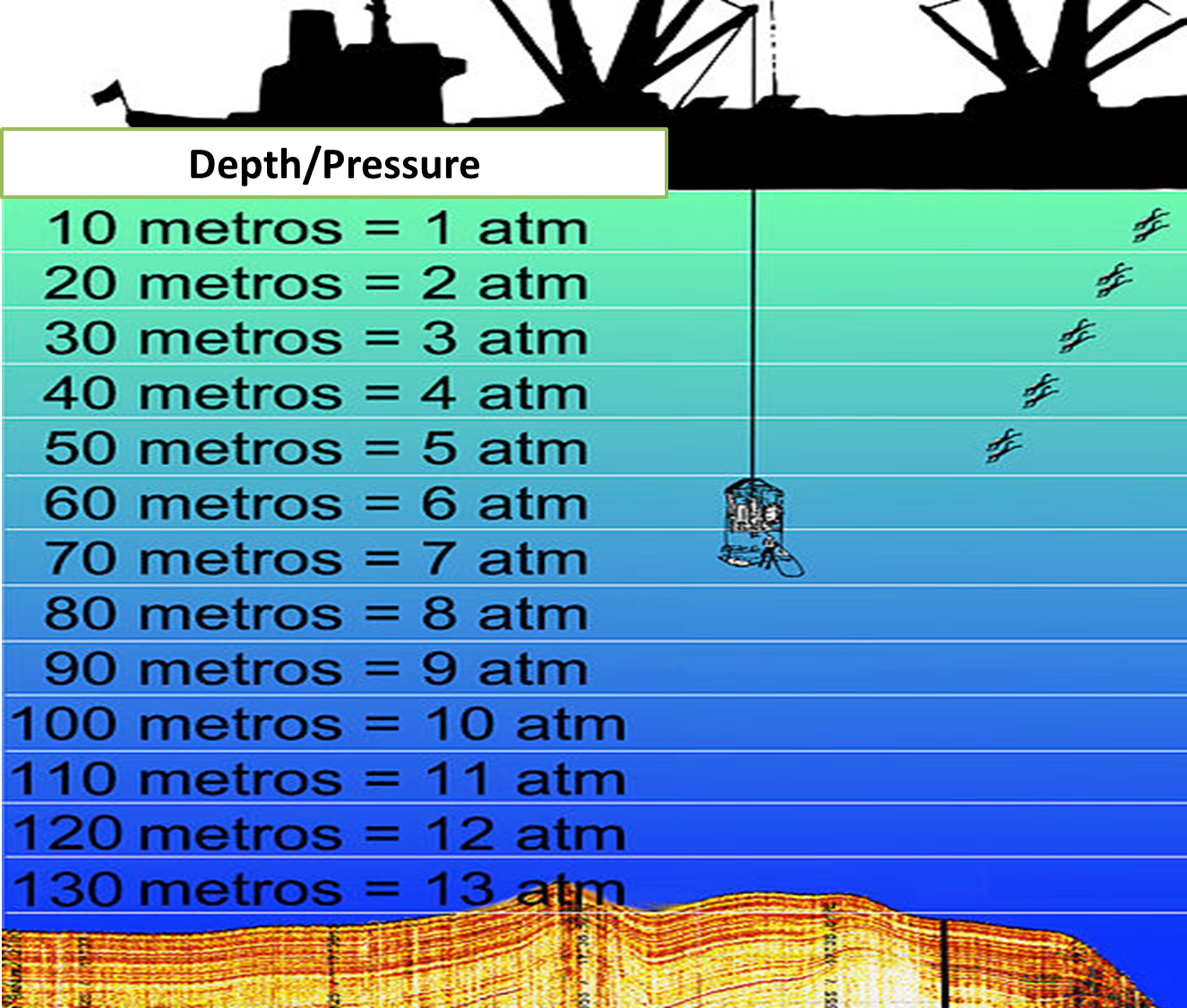
Fig. 6. Relationship between depth and pressure generated. Autor: Hannes Grobe/AWI
This assumed variation is responsible for the accidents that occur when diving without adequate measures. It is necessary to remember that the higher the pressure the gases are compressed and when an accelerated ascent to the surface causes the mixture of oxygen and nitrogen to become less dense and at this point it is less compressed, so that it exerts greater pressure on the walls of the lungs causing damage to it.
If the decompression is too high, parts of the gas molecules accumulate in the blood capillaries, preventing sufficient oxygen from reaching the brain and the person exhibits a behavior called air embolism that sometimes causes people to lose consciousness before reaching the surface, This phenomenon can be reversible but very painful, so the diver must spend an hour in a re-compression chamber whose objective is to reduce the size of the gas molecules, until they become harmless to the body.
Another aspect that we must take into account is the composition of oxygen we breathe, which is in concentration 20 - 80 with nitrogen, ie 20% is oxygen and 80% is nitrogen, so we can establish that it is a gaseous solution. In this sense, when the diver dives the water pressure on him is greater than atmospheric pressure, so our organs must balance and maintain a pressure equal to the pressure exerted by the water, for it has been developed specialized equipment that meet this task and which are decreasing the amount of oxygen that enters our body depending on the depth.
In other words, if the pressure exerted by the water is 2 atmospheres, the amount of oxygen entering our body will be 10%, an automatic readjustment performed by a special valve adapted to diving tanks.

Fig. 7. Relationship between depth and pressure generated. Autor: Alexander Lesnitsky
CONTRIBUTIONS FROM THE TOPIC
The approach to this topic allowed us to deepen our understanding of the laws of gases from Dalton's theory, as well as to understand the behavior of these substances as a function of atmospheric pressure and how these principles affect the health of people dedicated to diving as a recreational sport.
CONSULTED BIBLIOGRAPHY
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Katz Miguel John Dalton and the Atomic Theory. Epistemology and History of Chemistry - Course 2011. Artículo: Acceso Online
[3] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] WADE,LEROY. (2011). ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCACIÓN, MEXICO, 2011 ISBN: 978-607-32.()793•5. ÁREA: CIENCIAS
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
#posh twitter:
https://twitter.com/BGMadrid/status/1383257487458013188?s=20
Interesting article, I'm wondering how deep we can dive under the sea ?, I'm thinking about how Ahmed Gabr managed to set the record for diving 305 meters deep in the Red Sea in 2015.
Greetings @arnol99, the depth we can support depends on the diver's experience and physical condition, as well as the equipment he/she has. Thanks for stopping by and leaving your comment
That's a fascinating article, as a child I remember trying to breath through a pipe at the bottom of our backyard pool and I found it really hard to breath because of the pressure even just at 1m below the surface. I have always wondered how this occurs, yet divers can dive many metres and not have this problem. I goes to show you how much the pressure changes so quickly in water compared to air.
@elcomentador is a Comment Curation Project. We aim to reward those comments that add value to posts and encourage commenting on Hive.
If you want to learn more about our philosophy and how to qualify for a vote on our account, read our introduction post and join our Discord server where you can interact with other commenters, educate yourself on the art of commenting, learn about initiatives and much more.
Visit the hashtag #hivecomments and become an expert.
Hi @notak, no doubt chemistry allows us to substantiate even the smallest process. In the case of why the busos breathe without problems underwater, remember that the tanks have a valve that regulates the pressure and amount of oxygen that the diver needs and according to these requirements is the dose supplied. In your case, when you try to breathe without this scientific and technological implement, the results will be overwhelming. Thanks for stopping by and leaving your comment.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.