APPLIED CHEMISTRY/ CALCIUM CARBONATE (CaCO3) AS STRUCTURAL RAW MATERIAL OF EGG HULLS
(Edited)
Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of #Hive community, serve the present delivery to socialize through the community of @stemsocial, concerning the precipitation reactions applied to the formation of calcium carbonate (CaCO3) as structural raw material of the eggshells, where we will base chemically each one of the principles immersed in this process.
INTRODUCTION
Historically it has been documented that the formation of mineral material present in the earth has originated due to a cooling of the magma, although others of these minerals, arise due to the precipitation reactions, concept that we studied in previous deliveries and which we defined as:
Those substances that result from the combination of certain cations and anions in solution, generating water-insoluble substances that tend to become solid, which are deposited at the bottom of the container due to their own weight. [3]
In this sense, the precipitation reactions are of great importance at the biological level, especially in the production of minerals, specifically those involving calcium carbonate (CaCO3) whose function is defined in the formation of shells, teeth and fossils of some organisms.
Therefore, through this material we will make a tour of the precipitation reactions that give rise to the formation of calcium minerals, specifically regarding the structural aspects of the eggshells, as well as the chemical principles that are present in the process.
MIXES IN BALANCE AND THE PRINCIPLE OF LE CHATELIER
The synthesis of chemical products consists of achieving the maximum amount of products that the reagents can produce, with low energy consumption, although during the development of this process there are variables that cannot be totally controlled (temperature and pressure) so a readjustment must be made to maintain the balance of the reactions that are carried out.
Depending on the above, if we want the reactions to be completed with a maximum of performance, several factors can be altered to allow the achievement of such objectives. Among those that stand out:
1. Vary the concentration of the substances involved in the reaction (reagents and products).
2. Vary the temperature, volume and pressure of the system.
3. Adding a catalyst to decrease the activation energy of the system and thus accelerate it to achieve the desired balance.
The variation of the factors mentioned above, responds to the principle of Le Chatelier in which it establishes:
“If a disturbance to an equilibrium reaction mixture occurs, the net reaction advances in the direction that counteracts that disturbance" [3]
Natural systems do not escape this reality; under their autonomy they manage to maintain the aforementioned balance, making their processes more efficient, dynamic and balanced.
An example of the above is represented by the formation of the shells of some living beings. The same ones use the sea water, rich in ions calcium (Ca2+ ) and ions carbonates acid (HCO3-) to produce carbonate of calcium (CaCO3), material that some alive beings use in the production of their shells generating a habitat to survive.

Equation 1. Representation of calcium carbonate formation. Author: @madridbg, through Power Point 2010.

Fig. 3. Snail shell, structurally calcium carbonate. Author: István Mihály
CALCIUM CARBONATE (CaCO3) AND EGG HUSKS
The precipitation reactions are a sample of the dynamism that our planet presents in its constitution and evolution. An example of this is the structural formation of the egg shells, which needs an approximate demand of 125mg/hour of calcium ions (Ca2+ ), this demand represents an exhausting process for the hens.
In that sense, the same adaptive process allows that the necessary requirements arrive at the opportune moment, beyond, if it is counted on a diet high in carbonates that can be used in the formation of the shells.
If calcium intake from the diet is low, the hens' metabolism provides 10% of the accumulated requirements in the long bones of the hen's bone system in the form of calcium phosphate (Ca3(PO4)2.
Chemically the egg shells are formed by a crystalline form of calcium carbonate (CaCO3) which is called calcite. This process is carried out by an equilibrium precipitation reaction where calcium ions (Ca2+) and carbonate ions (CO3)2- travel through the bloodstream, reach the producing glands and precipitate where the shell is formed.
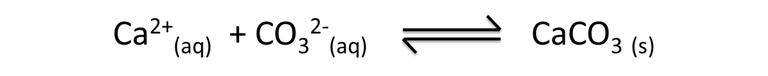
Equation 2. Representation of calcium carbonate in aqueous solution. Author: @madridbg, through Power Point 2010.
An interesting aspect that involves the concepts of equilibrium studied previously, is related to the formation of carbonate ions, a by-product obtained in the metabolic process of chickens. Carbonic anhydrase enzymes accelerate the reaction where carbon dioxide (CO2) is transformed into carbonic acid (H2CO3), according to the following reaction.
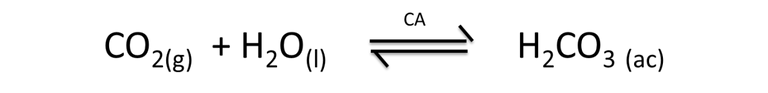
Equation 3. Representation of the formation of carbonic acid. Author: @madridbg, through Power Point 2010.
On the other hand, the carbonic acid (H2CO3) obtained is responsible for forming the greatest amount of carbonate possible so that it fulfills the desired structural function. However, if the diet provided to the hens is low in calcium the carbon dioxide-carbon acid balance (CO2)-(H2CO3), is broken and the body of the species must make a readjustment that responds to Le Chatelier's principle.
In this case, as a result of the work done, the body heats up and as the hens do not sweat they must pant to lower the body temperature, which produces a variation (decrease) in the concentration of carbon dioxide dissolved in the blood, breaking the desired balance and producing thin and fragile egg shells. This problem can be solved by supplying carbonated water to the hens in order to re-establish the balance as the concentration of carbon dioxide increases [2].
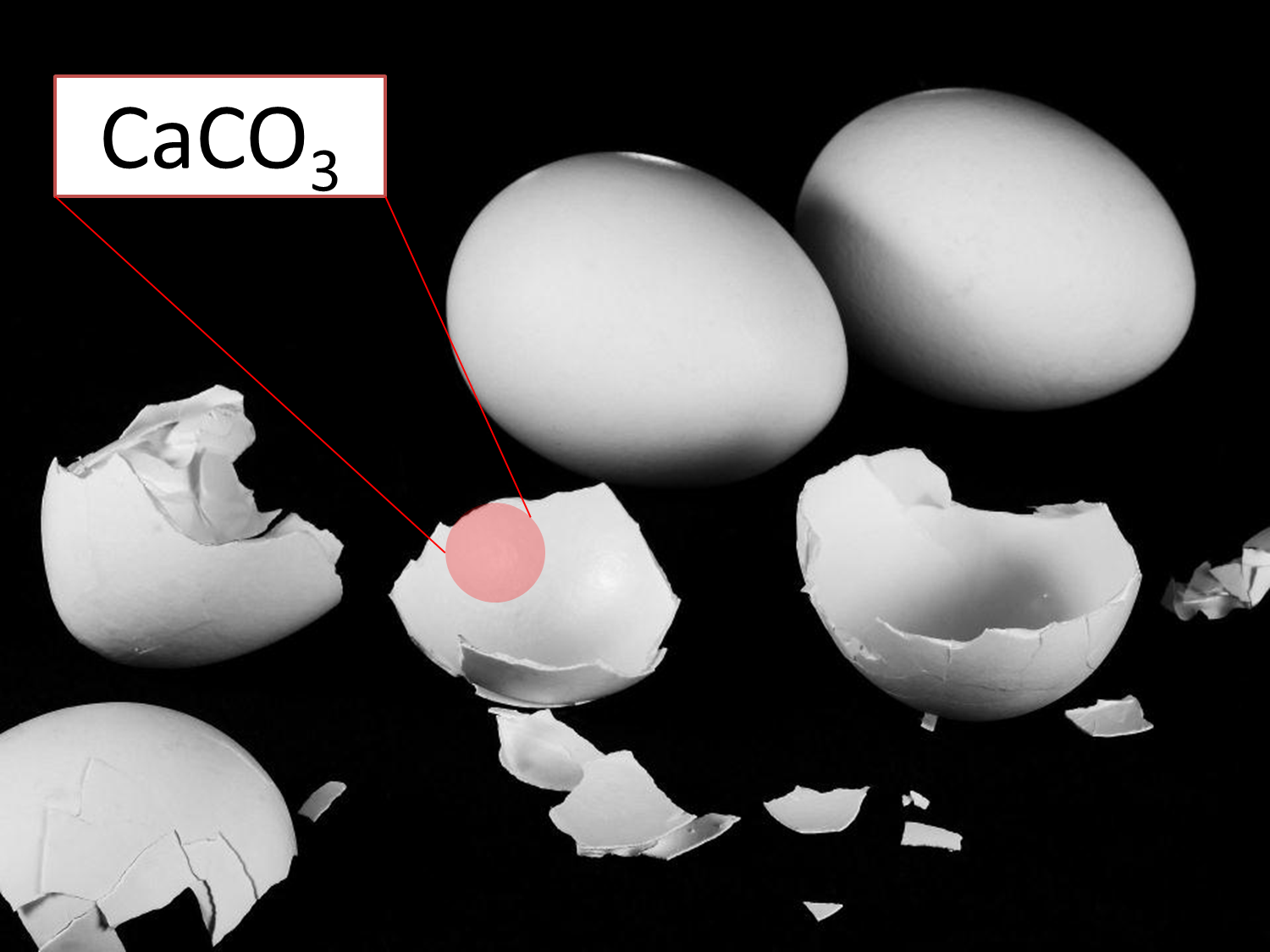
Fig. 4. Egg shells, structurally calcium carbonate. Author: @madridbg, Using background of Bruno /Germany
CONTRIBUTIONS OF THE THEME
Once again, we can visualize the dynamism presented by nature and how the scientific principles studied by man manifest themselves in the bilogical processes of the planet. In this sense, through the approach taken in this publication, the existing interaction between chemistry and the natural processes of the earth can be evidenced, which allows a contextualization of the scientific contents applied to biological processes.
BIBLIOGRAPHY CONSULTED
[1] Castillo, R and Ródenas, J. (2017). Analysis of the alterations of the hen's egg shell. NEREIS. 137-147. ISSN: 1888-8550. Article: Online Access
[2] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[3] Groel, N (2006). Generation of carbon dioxide by the reaction of an acid and a base Aquariological Silver Society . Article: Online Access
[4] Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2002) . Bioquímica. PEARSON EDUCACIÓN, S. A., Madrid, ISBN: 978-84-832-2694-0. Materia: Bioquímica, 577. ÁREA: CIENCIAS
[5] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[6] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[7] Salazar, G. (2008). Evaluation of the addition of organic minerals vrs. Inorganic minerals, on the external quality of eggshells in commercial caged laying hens. San Carlos University of Guatemala. Article: Online Access
[8] Valdés, J et al (2007). Egg shells: waste or added value for human health and poultry production? Natural Bioelements Research Center, Ministry of Public Health. Havana City, Cuba. Article: Online Access
[9] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
HTTP is in use instead of HTTPS and no protocol redirection is in place. Be careful and do not enter sensitive information in that website as your data won't be encrypted.
It's also a good habit to always hover links before clicking them in order to see the actual link in the bottom-left corner of your browser.
This auto-reply is throttled 1/20 to reduce spam but if it still bothers you reply "OFF HTTP". Or reply REVIEW for manual review and whitelisting.
https://twitter.com/BGMadrid/status/1346176781016051712
#posh twitter
https://twitter.com/BGMadrid/status/1346176781016051712?s=20
I like how you make chemistry totally relatable to a layman. Keep up the good work.
There's a typo in your third subheading; Calcium was written as Co instead of Ca.
Hello @gentleshaid it's nice to know that you liked the shared content. Thank you in advance for the observation at the typographical level, we have made the respective corrections. Greetings
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Greetings @steemstem, in advance thank you for the support and constant evaluation I receive from you in each of my publications
Your post has been voted as a part of Encouragement program. Keep up the good work!
Try https://ecency.com and Earn Points in every action (being online, posting, commenting, reblog, vote and more).
Boost your earnings, double reward, double fun! 😉
Support Ecency, in our mission:
Ecency: https://ecency.com/proposals/141
Hivesigner: Vote for Proposal
Hello @ecency. Thank you for your support and positive feedback on my publication