AMMONIA (NH3): RAW MATERIAL FOR CHEMICAL FERTILIZERS

Author: @madridbg, through Power Point 2010, using public domain images.
Welcome to all those readers of the #hive community. As it has been daily my publications are directed to the scientific area, with the purpose of maintaining the writing orientation that follows the community of @stemsocial who have kept themselves at the forefront in the topics associated with chemistry, biology, engineering, among others.
In this sense, in this opportunity we will make a survey on the use of Ammonia (NH3) in the preparation or elaboration of fertilizers, without leaving aside conceptual contents associated to chemistry that are present in this industrial process and that respond to stoichiometric units that allow us to quantify the yield of chemical reactions.
INTRODUCTION
Chemistry as a science in the course of time has evolved and has generated relevant changes in society, always having as its backbone the chemical reactions, understood as that process in which one or several substances change and produce new substances, whose properties are different from those of the starting compounds.
The agricultural, industrial, environmental and biological fields do not escape from this reality. In this sense, we say that we have life due to the set of reactions that occur within us and that allow us to generate the energy (ATP) necessary to carry out our daily tasks or for our organism to function.
In the agro-industrial field, the reactions play a preponderant role, because large quantities of fertilizers or substances are needed to provide the necessary nutrients to the soil and to be assimilated by the plants, which generates substrates with a high nutritional level that allow an adequate development of the vegetations.
Therefore, in this topic we will instruct the reader on the mechanisms present in the preparation of nitrogenous fertilizers, going through the general characteristics present in the stoichiometric equations.
REPRESENTATION OF CHEMICAL REACTIONS
In this section we will delimit the difference that exists between the reactions and the chemical equations. It is expected that the process where the changes of substance occur with modification of their properties, is known as chemical reactions, concept that was exposed in the previous section.
In that sense, during a chemical reaction we can use our senses and corroborate what is happening, because changes in color, temperature variation and even changes in the state of matter can be manifested. See figure 2.
Sometimes, none of the above mentioned signals are present, so it is necessary to perform a detailed analysis to predict whether a reaction has taken place

Fig. 2. Reaction between sodium bicarbonate and citric acid. Author: @madridbg, homemade experiment.
In figure 2, we can see the reaction that occurs between sodium bicarbonate (NaHCO3) and citric acid (C6H8O7), producing the release of carbon dioxide (CO2) responsible for the balloon inflation, in addition to generating water (H2O) and sodium citrate (Na3C6H507).
On the other hand, when we speak of a chemical equation we refer to a short symbolic group that allows interpreting the process of the reactions. The equations are composed of reagents, substance that will interact or react and products, those substances that are obtained as a consequence of the transformation of the reagents. To observe in detail the representation of a chemical reaction by means of the equations See figure 3.
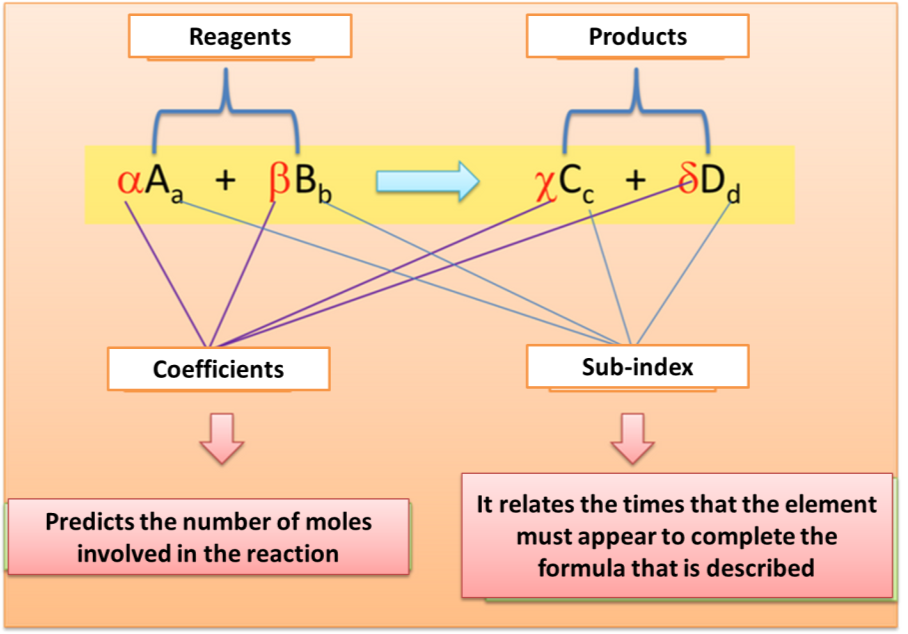
Fig. 3. Symbolic representation of a chemical reaction. Author: @madridbg, through Power Point 2010.
In order to instruct the reader in the behavior of the reactions in terms of a chemical equation, let's look at the following example:
Clothes bleach is a solution of sodium hypochlorite (NaClO), which is prepared by combining sodium hydroxide with chlorine. Represent the equation for this chemical reaction.

Fig. 4. Chemical equation implemented in the sodium hypochlorite preparation process. Author: @madridbg, through Power Point 2010.
From the previous representation, relevant information can be extracted about the chemical reaction that takes place, therefore we can establish that during the preparation of the bleach, 2 moles of sodium hydroxide (NaOH) react with 1 mol of chlorine (Cl2) to produce 1 mol of sodium hypochlorite (NaClO), 1 mol of sodium chloride (NaCl) and 1 mol of water (H2O).
In general terms, a chemical equation is nothing more than the paper representation of a real process called reactions that allows the operation and interpretation of the phenomenon that is occurring.
CHEMICAL FERTILIZERS: EQUATIONS AND THEIR STOICHIOMETRIC RELATIONS
The word stoichiometry comes from the Greek stoicheion, which means element. However, for practical reasons we will define it as the quantitative study of reagents and products in a chemical reaction. At an experimental and stoichiometric level, questions such as.
¿What amount of products are obtained if we make a specific amount of reagents react?
To give answers to such questions, we must know the relationships based on mol, grams, atoms and molecules. In this sense, to fulfill the established in the title of this work, we will focus on the relations based on moles.
In the same way in the stoichiometry of a reaction, it is necessary to know the reagent that limits the reaction (Limiting reagent), being understood as that substance that is exhausted first during the interaction of the reagents, therefore the percentage of yield of the product depends on this substance.
The behavior of the limiting reagent is exemplified below:
If we go to a hospital and we have to wait to be attended and in the waiting room there are five chairs and eight people. Only five people will be able to sit and the other three will be standing, which means that the number of people sitting is limited by the number of chairs present.
In other words, for the practical example my limiting substance or reagent is represented by the chairs and the people can be considered as excess substance or reagent. On an industrial level, it is necessary to know the terminology present in the chemical equations, as well as how a reaction works. Therefore, during the preparation of fertilizer, the concepts studied so far are applied.
It is necessary to remember, that to feed the world population and in accelerated growth, bigger and healthier crops or harvests are needed. That is where the importance of fertilizers lies, since every year thousands of tons of these products are added to the soil to improve its nutritional characteristics.

Fig. 5. Nitrogen fertilizer application to the soil. Lynn Betts
Depending on the subject, we will only focus on studying the fertilizers that have ammonium as their raw material (NH3), this does not imply that it is the only way or form to prepare them, since there is a diversity and types of these substances.
Ammonia is obtained by means of the Haber method, which consists of heating the mixture of hydrogen and nitrogen to 500 °C in the presence of iron and a small percentage of potassium and aluminium oxide, which act as catalysts for the reaction, that is, they accelerate it. According to the following equation.
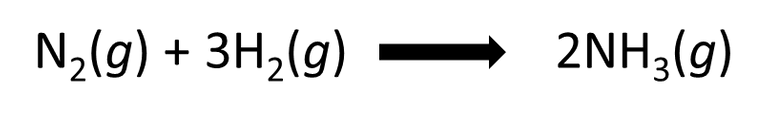
Fig. 6. Equation representing the preparation of ammonia. Author: @madridbg, through Power Point 2010.
Once the raw material is obtained, we can transform it and obtain sulphur, phosphorous or nitrogenous fertilizers.
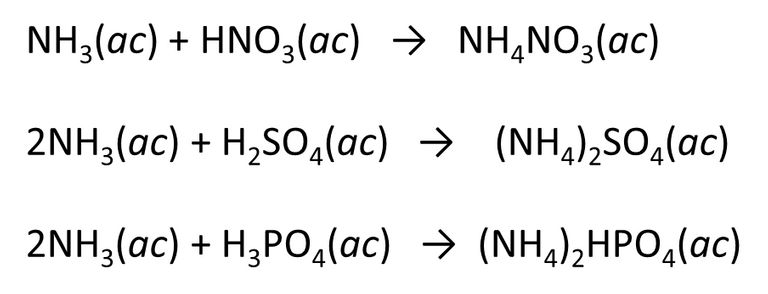
Fig. 7. Equation representing the preparation of nitrogenous fertilizers. Author: @madridbg, through Power Point 2010.
The choice of a fertilizer is influenced by different aspects that go from the cost, storage, transport, water solubility, among other factors that delimit the composition of a good product, as well as its availability in time.
PRACTICAL CONTRIBUTIONS OF THE SUBJECT
Once the theoretical part of the subject is finished, it is time to guide the reader in relation to the performance that we can obtain throughout a reaction, this task will be approached through a practical exercise.
Proposed exercise: Ammonium nitrate (chemical fertilizer), is prepared by the reaction of ammonia with nitric acid, according to the following reaction
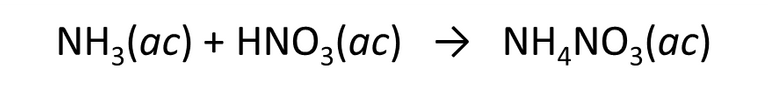
Fig. 8. Chemical equation implemented in the formation of ammonium nitrate. Author: @madridbg, through Power Point 2010.
In a technological process, 637.2g of NH3 is reacted with 1142g of HNO3. Determine:
1. Which of the reagents is the limiting factor?
2. What amount of NH4NO3 is obtained during the process
Solution:
Step 1: Extract the data provided by the statement
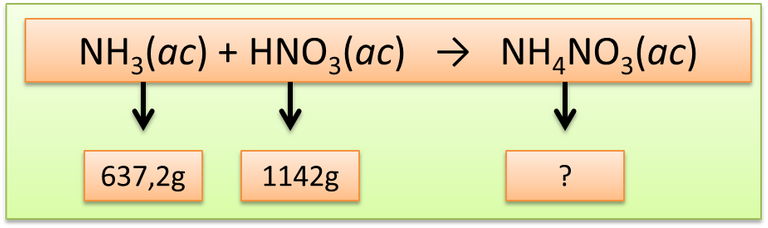
Author: @madridbg, through Power Point 2010.
Step 2: Calculate the molecular weight (PM) of the substances involved.
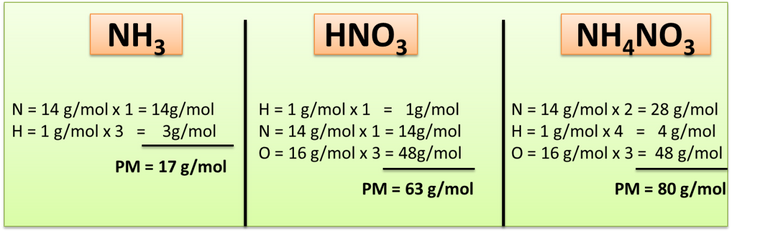
Author: @madridbg, through Power Point 2010.
Step 3: Transform the grams of the substances into moles using the molecular weight.
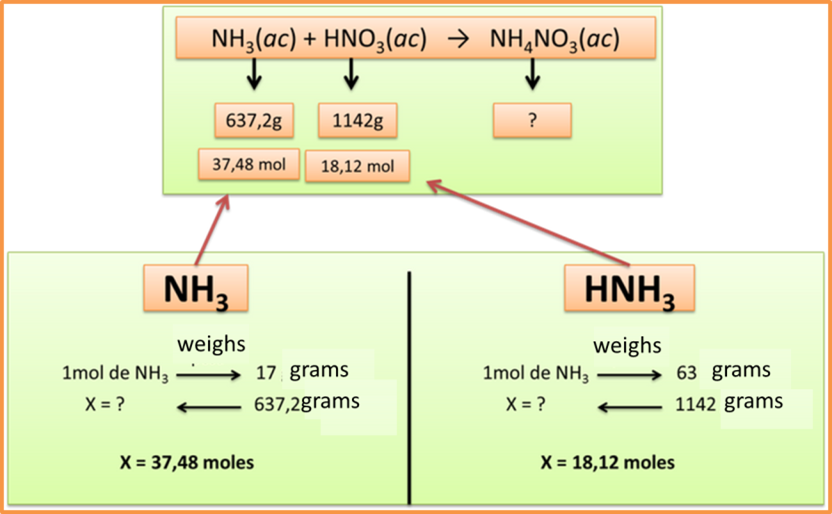
Author: @madridbg, through Power Point 2010.
Step 4: Establish stoichiometric ratios to determine the limiting reagent
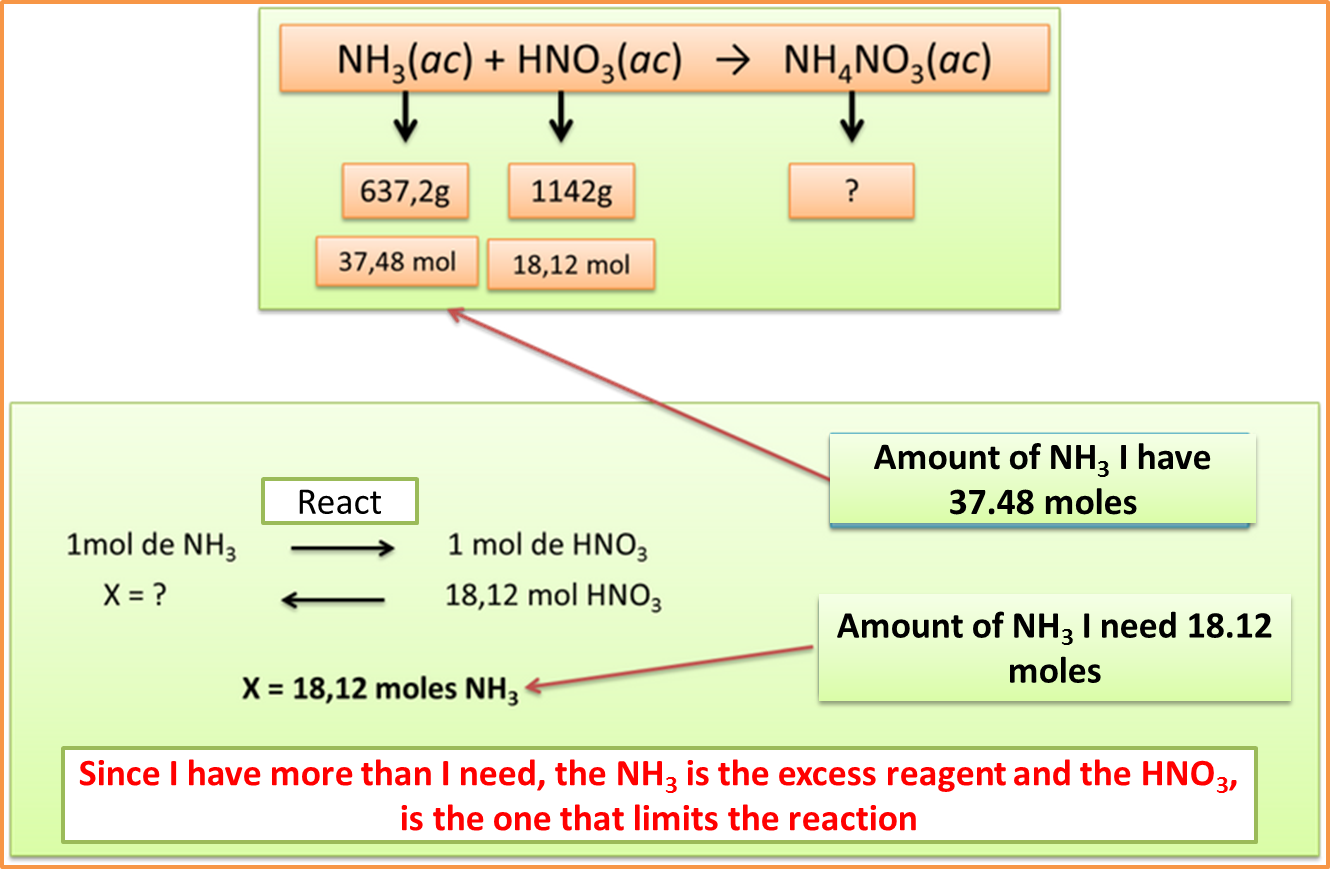
Author: @madridbg, through Power Point 2010.
Step 5: Determine the amount of product obtained based on the limiting reagent
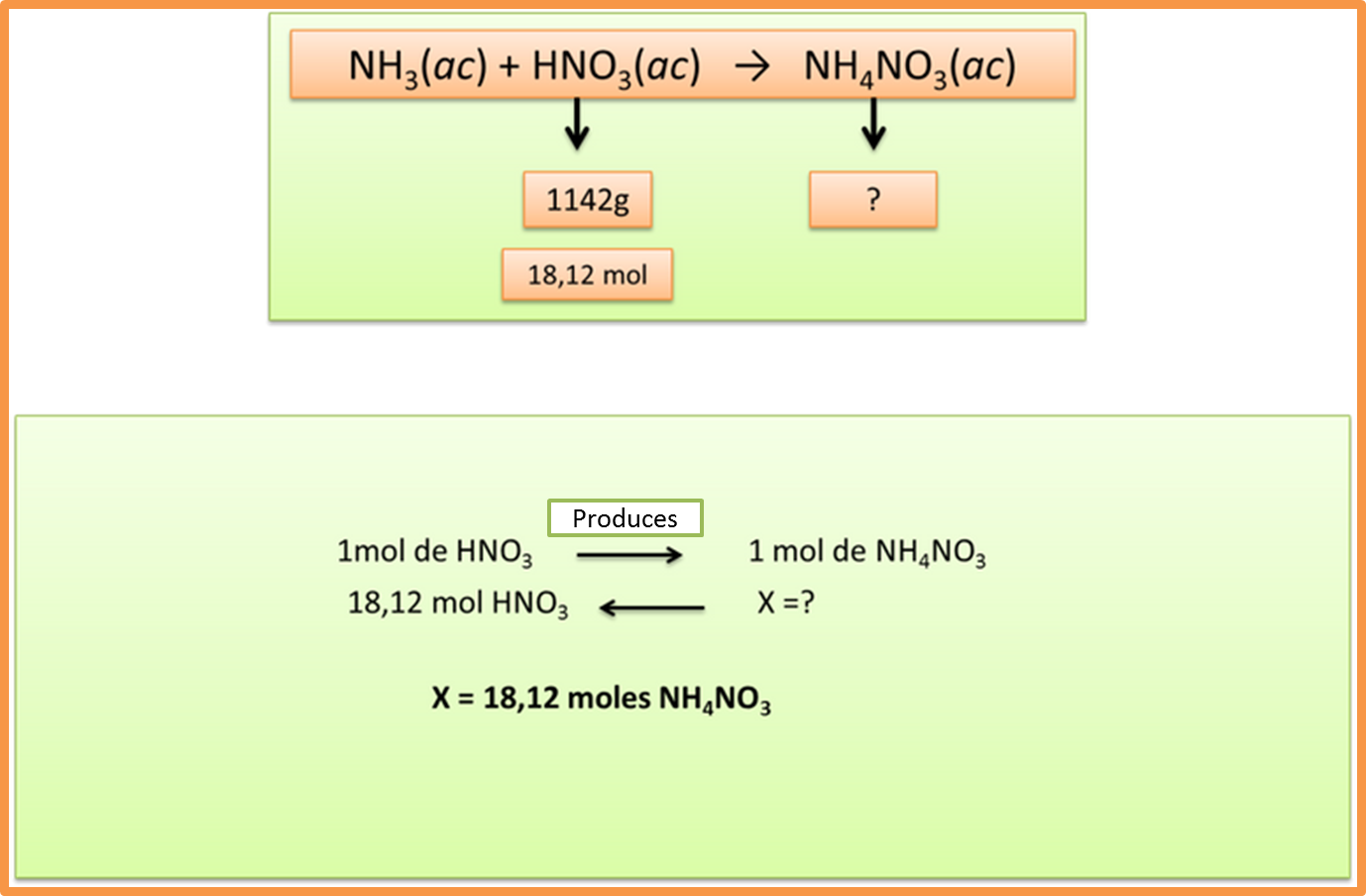
Author: @madridbg, through Power Point 2010.
Step 6: transform the moles of products obtained into grams
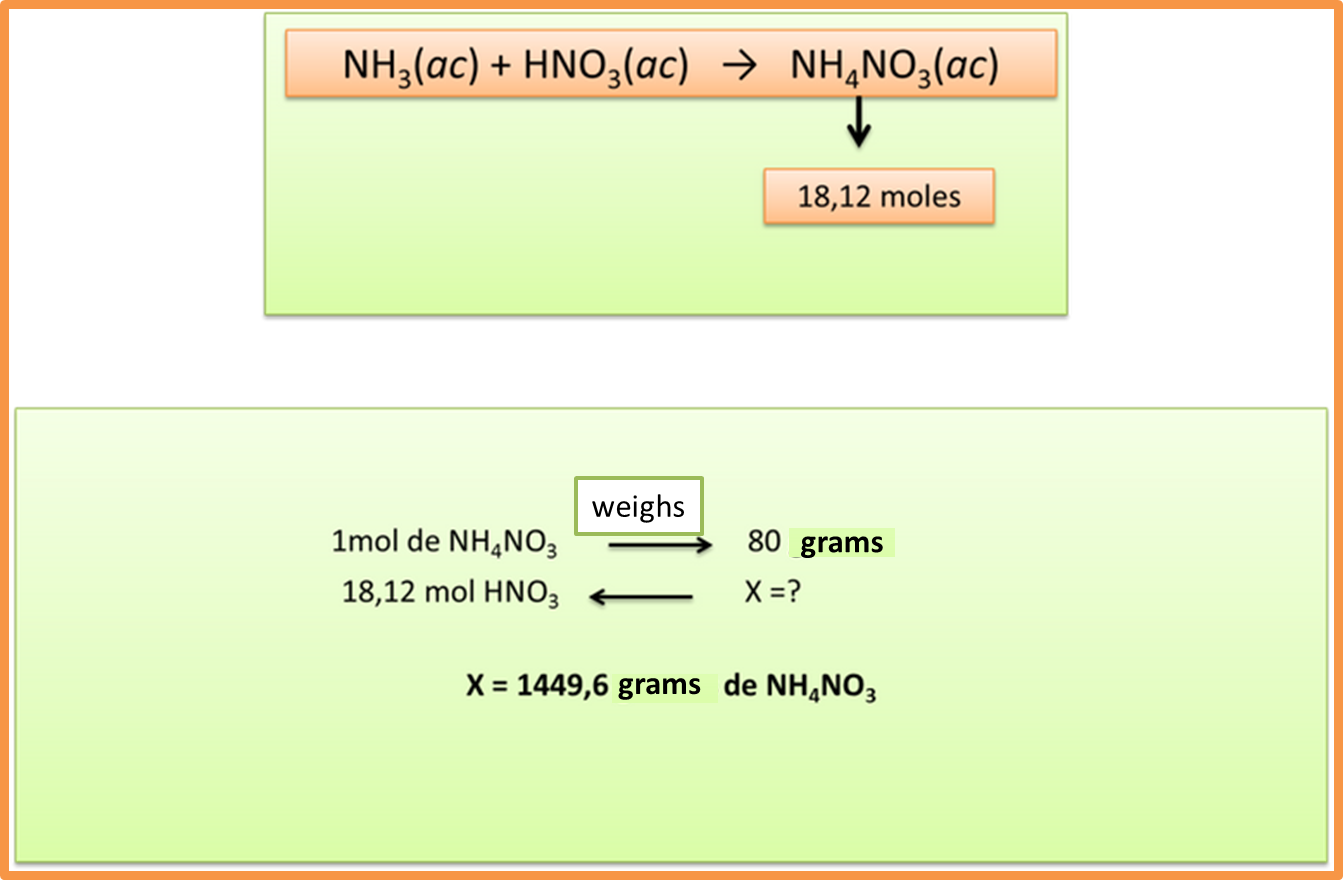
Author: @madridbg, through Power Point 2010.
We can determine that the implementation of this material allows the reader to know the theoretical behavior of the chemical reactions. Likewise, by contextualizing an applied technological process such as the preparation of fertilizers, we are consolidating the acquisition of quantitative operations related to the stoichiometry of chemical equations.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2002) . Bioquímica. PEARSON EDUCACIÓN, S. A., Madrid, ISBN: 978-84-832-2694-0. Materia: Bioquímica, 577. ÁREA: CIENCIAS
[3] McMURRY E., John y Fay C., Robert. (2008). Química general. Quinta edición PEARSON EDUCACIÓN, México, 2009 ISBN: 978-970-26 1286-5.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[5] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
#posh twitter
https://twitter.com/BGMadrid/status/1337936808005525504?s=20
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support. Using the STEMsocial app could yield even more supporti next time.