THE CHEMISTRY OF CARBOXYLIC ACIDS AND pH #4
Hello, my dear readers. This will be the last post of my series on THE CHEMISTRY OF CARBOXYLIC and pH, where I will be discussing the buffer solutions, carboxylates and the preparation of carboxylic acids.
BUFFER SOLUTIONS
A buffer solution resists changes in its pH when a small amount of either acid or alkali is added to it. A buffer solution is therefore used to control the pH of another solution to which a small amount of either acid or alkali is to be added. A typical buffer solution is a mixture of either a weak acid or its conjugate base, or a weak base and its conjugate acid. For example, aqueous solutions of carboxylic acid and its sodium or potassium salt will act as a buffer.
The chemical processes that take place in a solution containing a weak acid, HA and its sodium salt, NaA, will produce large concentrations of A- and HA:
HA(aq) ⇌ A- (aq) + H+ (aq)
This equilibrium lies almost entirely on the left. So, we can assume that the concentration of HA at equilibrium is that of undissociated HA. The sodium salt completely ionizes in water, to form A-(aq). Notice that this process is shown by a right-pointing arrow:
NaA(aq) → A- (aq) + Na+(aq)
We can therefore assume that a solution consisting of, for example, 0.100 mol dm-3 ethanoic acid and 0.500 mol dm-3 sodium ethanoate has a concentration of 0.100 mol dm-3 of undissociated acid, CH3COOH, and 0.500 mol dm-3 of ethanoate ion, CH3COO-.
What happens when an acid is added to a buffer solution?
When acid is added to a buffer solution, the extra H+ ions react with the conjugate base present, A+, to produce undissociated acid, HA. This is an example of Le Chatelier’s principle, since the equilibrium of the weak acid moves to the left to minimize the effect of the increase in the H+(aq) concentration. Since most of the extra H-(aq) added is converted into undissociated HA, the pH hardly decreases.
What happens when hydroxide ions are added to a buffer solution?
When extra alkali is added to a buffer solution, the extra OH- ions react with the H+ present to form water:
H+(aq) + OH-(aq) → H2O(l)
Extra H+ ions are provided by more of the weak acid as it dissociates to maintain the equilibrium between HA(aq), A-(aq) and H+(aq). The overall buffering action can be represented by:
HA(aq) + OH-(aq) → H2O(l) + A-(aq)
Calculating the pH of a buffer solution
Buffer solutions with different pH values can be prepared by changing the relative proportions of HA(aq) and A-(aq). It is easy to calculate the pH of a buffer solution provided the Ka of the weak acid and the concentrations of the weak acid and its conjugate base are known.
EXAMPLE
A buffer solution is 0.10 mol dm-3 in ethanoic acid and 0.20 mol dm-3 in sodium ethanoate. Calculate its pH.
ANSWER
Let x be the concentration of the H-(aq). We then have:
CH3COOH(aq) ⇌ CH3COO-(aq) + H+ (aq)
At start/mol dm-3: 0.10, 0.20, 0
At equilibrium/mol dm-3: (0.10 - x), (0.20 + x), x
We can assume that x is very small compared with 0.10 and 0.20, since ethanoic acid is a weak acid. Therefore, at equilibrium:
[CH3COOH(aq)] = 0.10 mol dm-3
[CH3COO-(aq)] = 0.20 mol dm-3
Substituting these values into the expression for the Ka for ethanoic acid:
Ka = [CH3COO-][H+]/[ CH3COOH] = 0.20 × x/0.10 = 2x
Now: Ka = 1.8 × 10-5
So: x = 9.0 × 10-6 mol dm-3
pH = -log10[H+(aq)]
= -log10(9.0 × 10-6)
= 5.0
The pH of the buffer solution is therefore 5.0 (to 2 sig. figs.).
Buffering in blood
To remain healthy, the acid-base balance of our blood has to be maintained at a constant pH of 7.4. If, for instance, the blood became acidic and this value dropped (as it does in a medical condition called acidosis), we would have to breathe rapidly to expel more of the acidic gas carbon dioxide. The main mechanism that maintains pH at 7.4 is the buffering action of several conjugate acid-base pairs – including H2CO3(aq) and HCO3- (aq), and H2PO4-(aq) and HPO42-(aq) – together with the buffering action of plasma proteins and haemoglobin. The action of the proteins is because of the carboxyl and amino groups in the side-chains of some of the amino acids that make up proteins. The concentrations of H2PO4-(aq) and HPO42-(aq) are too low for this conjugate acid-base pair to have a large buffering effect.
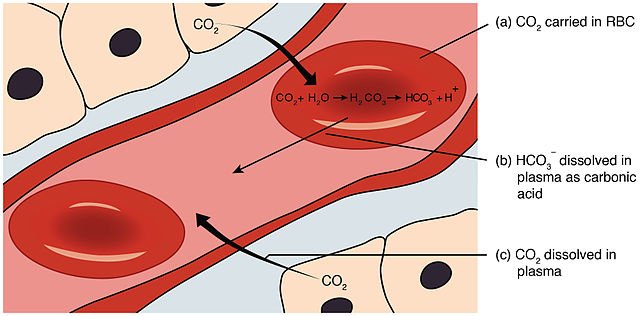
It is the equilibrium between carbon dioxide, carbonic acid, hydrogencarbonate ions and carbonate ions that acts as the most important buffering system in blood plasma:
H2CO3(aq) ⇌ HCO3-(aq) + H+(aq)
When the pH of blood decreases, the concentration of H+(aq) increases and the position of equilibrium shifts to the left to minimize this increase. The carbonic acid concentration does not rise indefinitely, since this too is in equilibrium with carbon dioxide and water, and so the concentration of dissolved carbon dioxide increases. Dissolved carbon dioxide is removed from blood by exchange in the lungs. When the pH of blood begins to rise, the H2CO3(aq)/HCO3- equilibrium shifts to the right to produce more H+(aq).
Strenuous exercise increases the metabolic rate which produces more carbon dioxide in tissue respiration. Representing the overall oxidation of glucose:
C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l)
Therefore, the concentration of dissolved carbon dioxide in the blood starts to increase and the net effect tends to lower the blood pH. However, this effect is counteracted by increase in breathing rate, to speed up gas exchange and thereby reduce the concentration of dissolved carbon dioxide. The blood pH therefore does not decrease.
CARBOXYLATES
Carboxylates are the salts of a carboxylic acid. They contain the carboxylate anion. Measurements have established that both the carbon-oxygen bonds in the carboxyl group have the same length, which is longer than the typical C=O bond, but shorter than the typical C-O bond. The reason they are not different lengths is that the negative charge on the anion is delocalized and does not reside on either of the two oxygen atoms.
Properties and uses of carboxylates
Most sodium and potassium carboxylates are soluble in water. How soluble they are depends on the length of the carbon chain of the carboxylate ion, and the solubility decreases as the chain becomes longer. As they are soluble, carboxylates are widely used in the food industry, which adds them to foods as preservatives and as acid regulators (buffers). As well as carboxylates, the food industry uses carboxylic acids. You can probably identify some of them in processed foods by their E numbers.
Sorbic acid, E200, which occurs naturally in some fruits, but is also made synthetically, is used as a preservative in such foods as soft drinks, cakes and frozen pizzas. Sodium E201, potassium E202 and calcium sorbate E203 are all used as preservatives. Benzoic acid, E210 occurs naturally in cherry bark, raspberries and tea, but normally made syntheticaly. It is used as a preservative and as an antioxidant in fruit products, soft drinks, pickles and salad dressings. Sodium E212, potassium E213 and calcium benzoate E214 are all used as preservatives while ethanoic acid, E260 is used in vinegar. Lactic acid, E270, found naturally in soured milk and yoghurt, acts as a preservative and a flavouring. Used in biscuits, confectionery and cakes.
PREPARATION OF CARBOXYLIC ACIDS
Carboxylic acids are either prepared by an oxidation reaction or by the hydrolysis of a derivative of a carboxylic acid.
Oxidation of primary alcohols and aldehydes
Carboxylic acids can be prepared by the oxidation of primary alcohols. For example, ethanol can be oxidized to give ethanoic acid by heating it under reflux with either acidified potassium dichromate(VI) or acidified potassium manganate (VII). In a similar way, aldehydes can be oxidized using the same reagents to form carboxylic acids. So phenylmethanal is oxidized to benzoic acid when refluxed with acidified potassium dichromate(VI).
HYDROLYSIS OF ESTERS
When an ester is refluxed with either dilute acid or dilute alkali, the ester is hydrolyzed to give the carboxylic acid or the carboxylate ion. For example, ethyl ethanoate can be hydrolyzed to give ethanoic acid (with acid hydrolysis) or sodium ethanoate (with aqueous sodium hydroxide). The sodium ethanoate can be converted into ethanoic acid by reaction with dilute hydrochloric acid.
HYDROLYSIS OF AMIDES
Amides can be hydrolyzed in the same way as esters. Again, there is the opportunity to use either acid- or base-catalysed hydrolysis. For example, ethanamide can be hydrolysed to give ethanoic acid and ammonium ion with acid hydrolysis, and ethanoate ion and ammonia with alkaline hydrolysis. Proteins and polypeptides are polymers that contain the amide linkage. They can be hydrolyzed either by refluxing with concentrated hydrochloric acid or by enzymatic action to give amino acids.
HYDROLYSIS OF NITRILES
Nitriles are hydrolyzed by refluxing in either dilute acid or aqueous alkali. Acid hydrolysis gives the ammonium ion and a carboxylic acid. Alkaline hydrolysis gives ammonia and the carboxylate ion. For example, ethanenitrile can be hydrolyzed by refluxing with hydrochloric acid to give ethanoic acid, but with aqueous sodium hydroxide it forms sodium ethanoate.
REACTIONS OF CARBOXYLIC ACIDS
The two most important synthetic reactions of carboxylic acids are the production of esters and the production of acyl chlorides.
ESTERIFICATION
When an alcohol and a carboxylic acid are refluxed together in the presence of an acid catalyst, such as concentrated sulfuric acid, an ester is produced. The reaction does not go to completion, but results in an equilibrium mixture that must be separated to isolate the ester. For example, ethanol, ethanoic and a trace of concentrated sulfuric acid give the ester ethyl ethanoate.
FORMATION OF ACYL CHLORIDES
In acyl chlorides (also known as acid chlorides), the carboxyl’s hydroxyl group is replaced by a chlorine atom. An acyl chloride is always named after the corresponding carboxylic acid. For example, the acyl chloride of ethanoic acid is called ethanoyl chloride and that of benzoic acid is called benzoyl chloride.
The conversion of carboxylic acids into acyl chlorides is very useful for the synthesis of esters and amides, because acyl chlorides are much more susceptible to nucleophilic attack than are carboxylic acids. Acyl chlorides are therefore used as synthetic intermediates.
Carboxylic acids are converted into acyl chlorides by reaction with sulfuryl (IV) chloride, SOCl2, or phosphorus (V) chloride, PCI5. Normally, the carboxylic acid is refluxed with the SOCl2, or PCl5, and the acyl chloride product is isolated by fractional distillation. For example, ethanoic acid can easily be converted into ethanoyl chloride, and benzoic acid into benzoyl chloride.
REDUCTION OF CARBOXYLIC ACIDS
Powerful reducing agents, such as lithium tetrahydridoaluminate, LiAIH4, reduce carboxylic acids to the corresponding alcohols (this is opposite to the preparation of carboxylic acids by oxidizing primary alcohols).
DECARBOXYLATION
Decarboxylation involves a reaction in which the carboxyl group is removed from a carboxylic acid, essentially as carbon dioxide, leaving behind a hydrocarbon. The reaction is typically carried out by strongly heating a mixture of powdered acid and sodium hydroxide. Alternatively the solid sodium carboxylate can be heated with solid sodium hydroxide. Typically the hydrocarbon formed, RH, will burn. If it burns with a smoky flame the R group probably contains a benzene ring.
Salt hydrolysis
A solution of sodium chloride is neutral and at 298 K has a pH of 7. This is typical behaviour of salts made from the reaction of strong acids with strong bases. A solution of sodium ethanoate is not neutral. It has a pH above 7 and is therefore an alkaline solution. This is typical behaviour of salts made from the reaction of weak acids with strong bases.
The explanation is due to a process called salt hydrolysis. In essence the following equilibrium is set up, which although it lies very much on the left-hand side does contribute to increasing the concentration of hydroxide ions.
CH3COO-(aq) + H2O) ⇌ CH3COOH(aq) + OH-(aq).
In water, sodium ethanoate is fully dissociated into Na+(aq) and CH3COO-(aq). The CH3COO-(aq) ions react reversibly with the small concentration of H+ naturally present in all water samples to form undissociated ethanoic acid, a weak acid. This in turn will affect the equilibrium involving the dissociation of water.
Since H+(aq) are used up the dissociation of water will move to the right to try and replace those lost. As a result there is an increase concentration of OH-(aq) and the solution becomes alkaline.
CONCLUSION
The summary of all these series of post on THE CHEMISTRY OF CARBOXYLIC AND pH; starting from 1, 2, 3, and this last post are the following.
- Brønsted-Lowry acids are proton donors and Brønsted-Lowry bases are proton acceptors.
- Strong acids are assumed to dissociate fully when dissolved in water. The dissociation of weak acids is an equilibrium process, in which the position of equilibrium lies very much to the side of the undissociated weak acid.
- Ka is the acid dissociation constant, which for the acid HA is given by the formula: Ka = [H+][A-]/[HA]
- pH is the negative logarithm to base 10 of the aqueous hydrogen ion concentration.
- A buffer solution resists changes in pH when small amounts of acid or alkali are added to it.
- Many buffer solutions are a mixture of an aqueous weak acid and its conjugate base.
- An indicator is a compound that changes colour in a solution at a certain pH value. Many indicators are weak acids.
- Carboxylic acids are typical weak acids; they react slowly with reactive metals such as magnesium to give hydrogen, react with bases to give carboxylate salts and release carbon dioxide from carbonates or hydrogencarbonates.
- Carboxylic acids can be prepared by the oxidation of primary alcohols or aldehydes by heating under reflux with acidified potassium dichromate(VI), or by the acid- or base-catalysed hydrolysis of esters, amides or nitriles.
- Carboxylic acids react with alcohols in the presence of an acid catalyst, such as concentrated sulfuric acid, to give esters.
Thank you for reading.
REFERENCES
https://www.britannica.com/science/carboxylic-acid
https://www.toppr.com/guides/chemistry/equilibrium/buffer-solutions/
https://en.wikipedia.org/wiki/Buffer_solution
https://byjus.com/chemistry/carboxylic-acid-properties/
https://study.com/academy/lesson/carboxylic-acid-structural-formula-properties-uses.html
https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/carboxylic-acids-and-their-derivatives/preparation-of-carboxylic-acids
https://www.britannica.com/science/carboxylic-acid/Synthesis-of-carboxylic-acids
https://www.publish.csiro.au/ch/pdf/CH9661013
https://aklectures.com/lecture/acyl-compounds-and-ester-hydrolysis/hydrolysis-of-amides
https://www.chemguide.co.uk/organicprops/nitriles/hydrolysis.html#:~:text=When%20nitriles%20are%20hydrolysed%20you,ammonium%20ethanoate%20going%20via%20ethanamide.
https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/carboxylic-acids-and-their-derivatives/reactions-of-carboxylic-acids
https://www.khanacademy.org/test-prep/mcat/chemical-processes/carboxylic-acids/a/carboxylic-acid-reactions-overview#:~:text=The%20carboxyl%20(COOH)%20group%20is,%3DO)%20and%20hydroxyl%20group.&text=In%20general%2C%20carboxylic%20acids%20undergo,by%20another%20nucleophile%20(Nu).
https://en.wikipedia.org/wiki/Ester
https://www.sciencedirect.com/topics/chemistry/esterification#:~:text=Esterification%20is%20the%20reaction%20of,Sciences%20and%20Chemical%20Engineering%2C%202014
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Acid_Halides/Synthesis_of_Acid_Halides/Preparation_of_Acyl_Chlorides
https://www.chemguide.co.uk/organicprops/acylchlorides/preparation.html
https://www.chemguide.co.uk/organicprops/acids/reduction.html
https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/carboxylic-acids-and-their-derivatives/reduction-of-carboxylic-acids#:~:text=Reductions%20of%20carboxylic%20acid%20derivatives,reduce%20carboxylic%20acids%20to%20alcohols.
https://en.wikipedia.org/wiki/Decarboxylation
https://greencamp.com/decarboxylation/#:~:text=Decarboxylation%20is%20the%20magic%20that,carboxyl%20g`roup%20and%20becomes%20psychoactive.
https://opentextbc.ca/chemistry/chapter/14-4-hydrolysis-of-salt-solutions/
https://courses.lumenlearning.com/cheminter/chapter/hydrolysis-of-salts-equations/
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.