THE CHEMISTRY OF CARBOXYLIC ACIDS AND pH #3
Hello dear readers, students and friends. Thank you all for the great motivation and support. Today's post will be a follow up on where I last stopped in my previous post on "THE CHEMISTRY OF CARBOXYLIC AND pH #2” In this new post, I will like you to know more about carboxylic acids and indicators.
CARBOXYLIC ACIDS
Carboxylic acids are weak acids. Therefore, they do not fully dissociate when dissolved in water. Many of them will not dissolve in water at all, although they can be shown to be acids since they react with alkali.

CARBOXYLIC ACIDS IN NATURE
Many naturally occurring organic compounds are carboxylic acids. Familiar examples already mentioned are citric acid in citrus fruits and ethanoic acid to vinegar. Three more examples are ethanedioic and lactic acid. Methanoic acid is the simplest carboxylic acid. Discovered in 1670, it was originally called formic acid, after the Latin word formicus for ant since it is one of the substances responsible for the sting of an ant bite.
THE CARBOXYL GROUP
A carboxylic acid contains the carboxyl group -COOH, which you may see written as CO2H. The carboxyl group contains a carbonyl bond C=O, and a hydroxyl bond O-H. Each bond is attached to the same carbon atom. This arrangement modifies the characteristic behaviour of both the carbonyl bond and the hydroxyl bond, which is why the carboxyl group is considered a functional group in its own right.
NAMING CARBOXYLIC ACIDS
Carboxylic acids are named by adding the suffix ‘oic acid’ to the name of the carbon chain. Unlike many functional groups, the carboxyl group contains one of the carbon atoms of the chain. The carboxyl group is almost always on the number 1 carbon atom, so this carbon atom rarely has to be given any other position number.
PHYSICAL PROPERTIES OF CARBOXYLIC ACIDS
Carboxylic acids are either solids or liquids at room temperature. The melting points of methanoic acid and ethanoic acid are quite high, and so it’s not unusual in cold weather for a bottle of concentrated ethanoic acid to be sold. In methanoic acid and ethanoic acid, the intermolecular forces are predominantly hydrogen bonds. However, as the carbon chain increases in length, there is also a considerable contribution from induced dipole-induced dipole attractions.
ACIDIC PROPERTIES OF THE CARBOXYL FUNCTIONAL GROUP
As mentioned already, carboxylic acids are weak acids, a property caused by the heterolytic fission of the hydroxyl bond in the COOH group. A carboxylic acid that can dissolve in water forms a weakly acidic solution in water. If a carboxylic acid cannot dissolve in water, it is almost impossible for any of its molecules to donate a hydrogen ion to a water molecule, and so the water remains about neutral. Nevertheless, insoluble carboxylic acids are acids, because they will react with bases, such as aqueous sodium hydroxide.
ACID-BASE REACTIONS OF CARBOXYLIC ACIDS
The typical reactions of aqueous acids arise from the presence of the aqueous hydrogen ion. Since carboxylic acids are weak acids, such reactions are slower than they are with strong acids, such as sulfuric acid. As the figure below shows, the acidic reactions of ethanoic acid always form the ethanoate ion. Remember that the volatility of carboxylic acids decreases as the relative formula mass increases. Remember also that liquids with low boiling point are very volatile, which means they have a high vapour pressure at room temperature. The solubility of carboxylic acids in water decreases as the relative formula mass increases. This is because the ability to form hydrogen bonds with molecules decreases as the non-polar alkyl group of the carboxylic acid gets longer.
REACTION OF CARBOXYLIC ACIDS WITH ALKALIS AND BASES
Carboxylic acids, such as ethanoic acid, can be neutralized by an alkali, such as aqueous sodium hydroxide:
CH3COOH(aq) + NaOH(aq) → CH3COO-Na+ (aq) + H2O(l)
This reaction is often carried out in the laboratory as a titration. An aqueous solution of the acid is added from a burette to a known volume of the alkali. At Neutralization, an indicator changes colour.
Bases such as magnesium oxide react slowly with aqueous carboxylic acids to form carboxylate salts. For example, dilute ethanoic acid reacts to form aqueous magnesium ethanoate:
MgO(s) + 2CH3COOH(aq) → Mg2+(CH3COO-Na+(aq) + H2O(l)
This reaction is much slower than the reaction of magnesium oxide with dilute hydrochloric acid because ethanoic acid is a weak acid and so has a much lower concentration of H+ (aq) than does hydrochloric acid, which is a strong acid.
Reaction with sodium carbonate and sodium hydrogencarbonate
Sodium hydrogencarbonate and sodium carbonate will react with aqueous solutions of carboxylic acids to form carbon dioxide:
NaHCO3(s) + CH3COOH(aq) → CH3COO-Na+(aq) + CO2(g) + H2O(l)
Na2CO3(s) + 2CH3COOH(aq) → 2CH3COO-Na+(aq) + CO2(g) + H2O(l)
TITRATION CURVES
The changes in pH can be monitored at regular intervals as an alkali is added to an acid. In titration, an alkali is added dropwise from a graduated burette to an acid of known volume and concentration, which contains an indicator. The addition continues until one drop changes the colour of the indicator, which signals that the alkali has just neutralized the acid.
Often, it is preferable to reverse the procedure, and add the acid to the alkali. The pH can then be plotted against the volume of alkali added, to obtain a graph known as a titration curve. The figure below is such a curve for the addition of aqueous sodium hydroxide to ethanoic acid. There are four types of titration curve, depending on whether strong or weak acids and bases are used.
Note that, as the alkali is added, the pH at first changes only slightly. Then, near the point of neutralization, it shoots up. With the addition of more alkali, it quickly levels off. The exact neutralization point, or equivalence point, can be estimated as the midpoint of the near-vertical portion of the graph, that is, where there is a large change of pH for a very small addition of alkali.
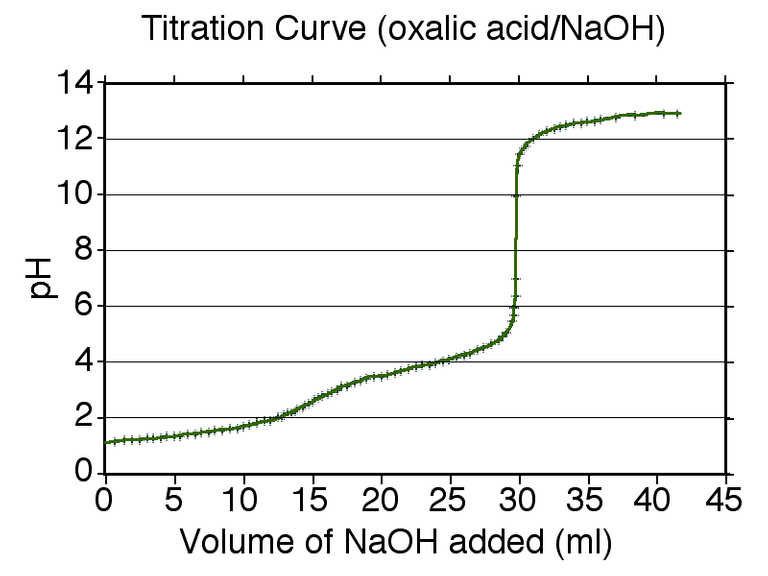
This is why you have to add the alkali one drop at a time when you are close to the end-point in a titration. An indicator is chosen that changes colour within the pH range of the vertical portion of the titration curve. However, as the graph shows, when a weak acid is titrated with a weak base, there is no sudden change in pH. This makes it very difficult to estimate the equivalence since any indicator changes colour within a range of pH values.
Indicators
The approximate pH value of a solution can be determined using an indicator solution, or indicator paper, and comparing the colour obtained against a colour chart. An indicator is a substance that changes colour when placed in an alkaline or acidic solution. The change of colour is because of changes in the complex organic structure of the indicator molecule, which affect the absorption of visible light by the molecule.
Litmus, a common indicator, is a natural product extracted from a lichen, while methyl orange, another common indicator, is a synthetic organic molecule known as an azo dye.
Indicators are often weak acids, and so in aqueous solutions they dissociate to a hydrogen ion and the conjugate base. It is Convenient to use the simplified formula Hln for an indicator, where ln stands for the conjugate base. In aqueous solution, the indicator dissociates:
Hln(aq) ⇌ H+(aq) + ln-(aq)
Normally, the indicator, Hln, has one colour and the conjugate base, ln, has another. In the case of litmus, Hln is red and ln is blue. In an acidic solution (one with an excess of aqueous hydrogen ions), the position of the equilibrium shifts to the left to minimize the effect of the increase of hydrogen ions.
So, as the concentration of Hln increases, the solution takes on the colour of the Hln molecule, which for litmus is red. This is an example of Le Chatelier’s principle. When an indicator is added to an alkaline solution, the position of equilibrium moves to the right, because H+(aq) reacts with the alkali. Hence, there is a large concentration of ln-(aq) and the solution takes on the colour of this species. The pH values for these colour changes vary with different indicators.
To be useful In titration, an indicator must change colour within the almost vertical part of the titration curve. Almost all indicators show the end point of a titration between a strong acid and a strong alkali. However, with a weak acid and a strong base, an indicator such as phenolphthalein should be used.

Determining pKa from a titration curve
To determine the Ka of a weak acid can be difficult. However, its pKa can be easily estimated by analysis of its titration curve. The pka is the ph value at the half-equivalence point, that is, the point at which only half of the volume of alkali needed to reach the equivalence point has been added.
The reason for this is that, at the half-equivalence point, concentration of the conjugate base, A- and that of the undissociated acid, HA, are almost equal. Therefore, they cancel out in the expression for Ka:
Ka = [H+(aq)][A-(aq)]/[HA(aq)]
So:
Ka = [H+(aq)] and pKa = pH.
Titration curves for diprotic acids
In a monoprotic acid, such as ethanoic acid, an acid molecule can donate only one proton to water. In a diprotic acid, such as ethanedioic acid, an acid molecule can donate two protons to water. Thus, ethanedioic acid has two Ka values, designated Ka1 and Ka2. In their significance, Ka1 refers to equilibrium 1 and Ka2 to equilibrium 2. The titration curve for a diprotic weak acid has two equivalence points.
Enthalpy of neutralization
The enthalpy change of neutralization is defined as the enthalpy change when one mole of water is produced during the neutralization of an aqueous acid with an aqueous alkali. The neutralization of an acid with an alkali is exothermic and represents the reaction between an aqueous hydrogen ion and an aqueous hydroxide ion:
H+(aq) + OH-(aq) → H2O(l)
This reaction is common to all acid-alkali neutralization reactions, so by Hess’s law, the enthalpy of neutralization should be independent of the acid and the alkali used. When a strong acid is neutralized by a strong alkali the enthalpy of neutralization is -57 KJ mol-1, which does not change even if the strong acid or strong base is changed. This value is considerable smaller, however, it changes when a weak acid or a weak base is neutralized. The reason is quite simple – a weak acid is not fully dissociated, so that before the neutralization takes place the acid must dissociate and dissociation is an endothermic process. So the enthalpy of neutralization in this case has the exothermic contribution of the neutralization and an endothermic contribution needed to dissociate the acid or the base
I will continue the rest of the topic, such as the Buffer solution and the carboxylates, in my next post. Thanks for reading.
REFERENCES
https://en.wikipedia.org/wiki/Carboxylic_acid
https://www.britannica.com/science/carboxylic-acid
https://www.britannica.com/science/carboxylic-acid
https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/carboxylic-acids-and-their-derivatives/reactions-of-carboxylic-acids
https://www.khanacademy.org/test-prep/mcat/chemical-processes/carboxylic-acids/a/carboxylic-acid-reactions-overview
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Module
https://www.bbc.co.uk/bitesize/guides/zmmncqt/revision/2
http://www.docbrown.info/page04/OilProducts10.htm
https://www.khanacademy.org/test-prep/mcat/chemical-processes/titrations-and-solubility-equilibria/a/acid-base-titration-curves
https://www.britannica.com/science/chemical-indicator
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules
https://sciencing.com/calculate-pka-titration-7834752.html
http://blamp.sites.truman.edu/files/2012/03/222-Determination-of-pKa-from-Titration-Curve.pdf
https://www.chemguide.co.uk/physical/acidbaseeqia/phcurves.html
https://crippen.education.ufl.edu/calculators/activities/DiproticAcid.html
https://www.saddleback.edu/faculty/cabel/Saddleback/Chem_1B_Schedule_files/exp9-polyprotic-titrations-sp20.pdf
https://www.ccri.edu/chemistry/courses/chem_1100/wirkkala/labs/Enthalpy_of_Neutralization.pdf
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Congratulations @empressteemah! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!