THE CHEMISTRY OF CARBOXYLIC ACIDS AND pH #2
Hello, dear readers, colleagues, students and friends. This post is sequel to my previous post on THE CHEMISTRY OF CARBOXYLIC ACIDS AND pH, where I extensively discussed the effect of pH balance in blood, foods and industry and also on acid and its various types. Today, I am writing on acid dissociation constant, Ka, pKa, the pH and solving some calculation examples.

Ben Mills, Public Domain
ACID DISSOCIATION CONSTANT
Once a weak acid has dissolved in water, it quickly reaches the state of dynamic equilibrium. It is possible to gauge just how weak an acid is by determining the acid dissociation constant, Ka. The a in K, stands for 'acid'. A weak acid HA dissociates in water and reaches a state of dynamic equilibrium:
HA(aq) ⇌ H+(aq) + A-(aq)
The acid dissociation constant is given by:
Ka = [H+(aq)][A-(aq)]/[HA(aq)]
The square brackets represent the concentrations of the various particles measured in mol dm-3. For example, [H+ (aq)] is the concentration of aqueous hydrogen ions in mol dm-3. Note that the concentrations used are those at the equilibrium position.
The acid dissociation constant for ethanoic acid is:
Ka = [H+(aq)][CH3COO-(aq)] / [CH3COOH]
Units = (mol dm-3)(mol dm-3) / (mol dm-3)
The smaller the value of the acid dissociation constant, the weaker the acid. Ethanoic acid, CH3COOH, has a Ka of 1.8 × 10-5 mol dm-3, whereas nitrous acid, HNO3, has a Ka of 4.5 × 10-4 mol dm-3. Notice that the acid dissociation constant includes a unit. It is not just a numerical value. Remember that the acid dissociation constant is a modified equilibrium constant.
pKa
The numerical values of Ka for strong acids normally lie in the range from 10-1 to 102, but for weak acids they are below 10-4. To avoid dealing with the small numbers associated with weak acids, we use pKa. This is its definition: pKa is the negative logarithm to base 10 of the acid dissociation constant: pKa = -log10Ka
Note that as the acid strength decreases, pKa increases. So a high pKa value means a very weak acid. Also pKa values are easier to compare than numerical Ka values, since they are not very small numbers expressed in standard form (log to base e). All scientific calculators have a key labelled lg or log. Do not use the key labelled ln, which is the 'natural logarithm'.
PeterGans, Public Domain
EXAMPLE
The acid dissociation constant for nitrous acid is 4.5 × 10-4 mol dm-3. What is the pKa value for nitrous acid?
ANSWER
pKa = - log10Ka
Substituting the value for Ka gives:
pKa = -log10(4.5 × 10-4)
pKa = 3.3
Propanedioic acid has two pKa values, because one molecule can donate two hydrogen ions. The first is donated more easily than the second:
HOOCCH2COOH(aq) ⇌ HOOCCH2COO- (aq) + H+(aq); pKa = 2.77
HOOCCH2COO- (aq) ⇌ -OOCCH2COO- (aq) + H+(aq); pKa = 5.66
Why is sulfuric acid strong and ethanoic acid weak?
The ability of a carboxylic acid, such as ethanoic acid, to donate a hydrogen ion depends on the strength of the O-H bond in the carboxyl group. Likewise the ability of sulfuric acid to donate a hydrogen ion depends on the strength of its O-H bonds. In simple terms, the stronger the O-H bond(s), that is, the greater the bond energy, the weaker the acid. In sulfuric acid the presence of many highly electronegative oxygen atoms withdraws electrons from the O-H bond and makes it weaker than the O-H bond in ethanoic acid. This is not the whole story, since the stability of the conjugate bases formed is also important. So the sulfate ion and the hydrogensulfate ion are more stable conjugate bases than the ethanoate ion.
pH
An acid is a proton donor – when it dissolves in water the H+(aq) concentration increases. That is, the higher the H+(aq) concentration, the more acidic solution becomes. However, the problem in measuring hydrogen ion concentration is that for many solutions it is extremely small numerically. For example, the hydrogen ion concentration in wine is only about 5 × 10-4 mol dm-3. In blood, it is even smaller, being about 4 × 10-8 mol dm-3.
OpenStax College, CC BY 3.0
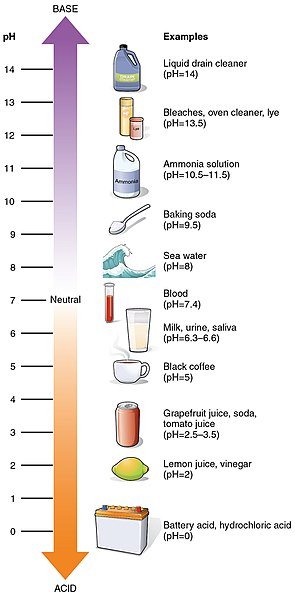
In 1909, the Danish chemist Sören Sörensen, working for the brewers Carlsberg, suggested an alternative way to measure the acidity of a solution – the pH scale.
The pH scale is constructed from the negative of the logarithm to base 10 of the [H+(aq)]. Thus, aqueous hydrogen-ion concentrations that may differ by, say, a hundred million million, are converted into numbers on a scale which runs from just below 0 to just above 14, Accordingly, the pH of a solution is described by the equation:
pH = -log10[H+(aq)]
where the aqueous hydrogen ion concentration is in mol dm-3
Measuring the pH of a solution
Our sense of taste is very sensitive to pH, and it provided the earliest way to distinguish between acids and alkalis. We can detect a sour acidic taste in solutions whose pH values are as high as 5, which corresponds to H+(aq) concentrations as low as 10-5 mol dm-3. The familiar acidic taste of most fruit juices and soft drinks is caused by the presence of weak acidic, which give these drinks a pH value of about 3.
When the pH is below 2, we find the taste distinctly unpleasant and the liquid is sufficiently acid to burn the lining of the mouth. In the same way, we can detect alkalis with a pH value of 9 to 10 as they taste bitter. Liquids with higher pH values than this are dangerous to taste and also burn the lining of the mouth.
The pH of a solution is measured accurately using a pH meter, which converts the difference in electrical potential between two electrodes into a pH reading. An indicator, such as universal indicator, which gives a range of colour in solutions of different pH values, can be used to estimate the pH value of a solution.
CALCULATING THE pH OF STRONG ACIDS
To calculate the pH of a strong acid, substitute the appropriate H+(aq) concentration in the equation for pH. This can be deduced from the concentration of the acid itself, since it is assumed that every acid molecule has dissociated. It is also possible to calculate the H+ concentration given the pH.

André Karwath, CC BY-SA 2.5
EXAMPLE 1.
What is the pH of a solution of 1.00 mol dm-3 sulfuric acid?
ANSWER
In water, sulfuric acid is assumed to be fully dissociated because it is a strong acid:
H2SO4 → 2H+ + SO42-(aq)
From the equation, every mole of H2SO4 gives 2 moles of H+ (aq). The concentration of 1.00 mol dm-3 refers to moles of the undissociated H2SO4, so the concentration of H+(aq) must be twice this. Therefore,
[H+(aq)] = 2 × 1.00 mol dm-3 = 2.00 mol dm-3
Substituting this value in the equation for pH gives:
pH = -log10 [2.00] = -0.301
NOTE: A negative pH value means that the concentration of aqueous hydrogen ions, [H+(aq)], in mol dm-3, is very high.
EXAMPLE 2.
A sample of acid rain has a pH of 3.41. What is the molar concentration of hydrogen ions?
ANSWER
The relationship between pH and [H+(aq)] is:
pH = -log10[H+(aq)]
Substituting the pH value gives
3.41 = -log10[H+(aq)]
So -3.41 = log10[H+(aq)]
So log -3.41 = [H+(aq)]
Using a calculator [H+(aq)] = 3.39 × 10-4 mol dm-3
The pH of boiling water
At 298 K, the pH of pure water is 7.00, which is designated neutral on the pH scale. So the concentration of H+(aq) in pure water is 1.00 × 10-7 mol dm-3. An acid has a pH below 7. That is, the concentration of H+(aq) is greater than 1.00 × 10-7 mol dm-3.
When a pH meter is placed in boiling pure water, the reading is pH 6.12. So, apparently, boiling water is acidic. But how can it be? In boiling water, the concentration of H+(aq) has increased, but so has the concentration of the aqueous hydroxide ion. So, there should be no surplus of either type of ion:
H2O(l) ⇌ H+(aq) + OH-(aq)
Now, this is the same situation as that in water at 298 K, which is considered to be neutral. The paradox is easy to explain. In boiling water, a greater proportion of water molecules dissociates at any one time, but there is no surplus of hydrogen ions.
CALCULATING THE pH OF WEAK ACIDS
Ethanoic acid has already been described as a weak acid that reaches a state of dynamic equilibrium when dissolved in water. When this state is reached, the concentration of aqueous hydrogen ions is very low compared with the concentration of the undissociated ethanoic acid. We can calculate the pH of aqueous ethanoic acid provided that we know the numerical value of the acid dissociation constant, or the percentage of ethanoic acid molecules that have dissociated.
EXAMPLE
What is the pH of 0.10 mol dm-3 ethanoic acid, given that 1.3 per cent of the ethanoic acid molecules have dissociated?
ANSWER
In water, ethanoic acid forms an equilibrium mixture very quickly:
CH3COOH(aq) ⇌ H+(aq) + CH3COO-(aq)
The 0.10 mol dm-3 is the concentration of CH3COOH before any has dissociated.
Given that only 1.3 per cent of ethanoic acid molecules dissociate, then the concentration of ethanoic acid that has dissociated must be 1.3 per cent of 0.100 mol dm-3 which is 1.3 × 10-3 mol dm-3.
From the equation, every mole of CH3COOH(aq) that dissociates gives 1 mole of H+ (aq). So, [H+(aq)] must be 1.3 × 10-3 mol dm-3.
Substituting this value into the equation for pH gives:
pH = -log10 (1.3 × 10-3) = 2.9
So, that will be all on the segment of acid dissociation constant, Ka, pKa, and pH. In my next post, I will discuss more about carboxylic acids and the carbonyl group.
Thanks for reading.
REFERENCES
https://en.wikipedia.org/wiki/Acid_dissociation_constant
https://www.bbc.co.uk/bitesize/guides/zcjjfcw/revision/7
https://en.wikipedia.org/wiki/Acid_strength
https://www.chemguide.co.uk/physical/acidbaseeqia/acids.html
https://en.wikipedia.org/wiki/PH
https://www.sciencedirect.com/topics/engineering/ph-of-a-solution
http://hyperphysics.phy-astr.gsu.edu/hbase/Chemical/ph.html
https://sciencing.com/calculate-ph-strong-acid-6392888.html
https://www.thoughtco.com/calculating-ph-of-a-strong-acid-problem-609587
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736366/
https://www.toppr.com/ask/question/the-ph-of-boiling-water-is-64-this-implies-that/
https://www.chemguide.co.uk/physical/acidbaseeqia/acids.html
https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Chem1_(Lower)/13%3A_Acid-Base_Equilibria/13.03%3A_Finding_the_pH_of_weak_Acids%2C_Bases%2C_and_Salts
https://www.thoughtco.com/calculating-ph-of-a-weak-acid-problem-609589
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app and including @stemsocial as a beneficiary, which give you stronger support.
Congratulations @empressteemah! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!