THE CHEMISTRY OF CARBOXYLIC ACIDS AND pH
Hello readers, colleagues, students and friends. Thank you all for the great feedback and support. Today, I am starting a new series of post tagged, “THE CHEMISTRY OF CARBOXYLIC AND pH”.
But I will like to introduce the post briefly by explaining the effect of pH in our blood, food and in the industry, before I then go further in explaining more about an acid and its different types.

pH balance in blood, foods and industry
Blood is very intimately connected with the biochemistry of all our tissues, which function correctly only while the blood is maintained at pH 7.4. In fact, death is likely to follow if the blood pH departs from this value for any length of time.
Chemical reactions in the blood itself continually produce acids and alkalis, which would seriously upset the pH level but for the fact that blood also contains buffers. These chemicals resist changes in blood pH and keep its fluctuations within safe limits.
Just as our bodies need a steady pH, so we take steps to control the pH of a wide range of foods and drinks we consume, for their stability as well as for our taste preferences and health. Wines, beers and lagers contain acids, such as ethanoic acid and tartaric acid. On the one hand, acidity helps to preserve them, but on the other too much acid makes them sour and vinegary, so food chemists ensure that the pH strikes the right balance.
When designing new chemical processes, too, industrial chemists often need to find ways to stabilize the pH values of solutions so that they will not significantly change when small amounts of acid or alkali are added. Again, for this buffers are required: often these are carboxylic acids and their soluble salts, the carboxylates, the subjects of this chapter.
SO, WHAT IS AN ACID?
Carboxylic acids are organic chemicals that contain a group of atoms known as the carboxyl group, COOH. Many common chemicals, such as citric acid in lemon juice and ethanoic acid in vinegar, are carboxylic acids and are described as weak acids.
So before we go into the chemistry of carboxylic acids, we need to be clear what is meant by the term 'acid' and why chemists talk of 'weak acids' and 'strong acids'.
Chemists knew about the properties of acids long before they understood what makes an acid. In the late 1700s, they believed that oxygen was the essential element in acids. Then in 1810, Humphry Davy disproved this hypothesis when he discovered that hydrochloric acid consists of only hydrogen and chlorine. So, hydrogen was established as the element common to all acids.
One early definition says that an acid is a compound whose molecule has at least one hydrogen atom that can be replaced by a metal atom. For example, hydrochloric acid, HCl (aq), forms sodium chloride, NaCl, when hydrogen is replaced by sodium. In the same way, ethanoic acid, CH3COOH(aq), is an acid because it forms sodium ethanoate, CH3COONa(aq).
However, as theoretical chemistry developed, more sophisticated generalized definitions of an acid – and a base – were introduced. CH3COONa(aq) is an ionic salt and is sometimes written as CH3COO-Na+.
BRØNSTED-LOWRY THEORY
In 1923, the Swedish chemist Johannes Brønsted and the English chemist Thomas Lowry defined acids and bases thus: An acid is a proton donor and a base is a proton acceptor. According to this definition, when an acid reacts with a base, a proton is transferred from the acid to the base.

ACID-BASE REACTIONS
Put simply, an acid-base reaction is one in which an acid is neutralized by a base, or vice versa. During an acid-base reaction, a proton is transferred from the acid to the base. The Brønsted-Lowry theory of acids and bases really focuses on the behaviour of acids and bases in water. The water molecule is amphoteric, which means that it can behave as either an acid or a base. A proton remains when a hydrogen atom loses an electron. So a proton is the same as a hydrogen ion, H+. An alkali is just a base that dissolves in water. When an acid dissolves in water it produces hydrogen ions in solution.
BEHAVIOUR OF BRONSTED-LOWRY ACIDS IN WATER
When an acid, HA(aq), is added to water, a proton is transferred to a water molecule:
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
In H3O+(aq) + A- (aq), a single water molecule forms a dative covalent bond with a hydrogen ion. It is called an aqueous hydrogen ion or an oxonium ion. (The term hydronium ion is also sometimes used.)
So when, for example, pure nitric acid is added to water, there is an immediate reaction in which a proton is donated from the acid to a water molecule:
HNO3(aq) + H2O(l) → H3O(aq) + NO3-(aq)
Be careful with the use of the formula HCl. The formula HCl(g) is hydrogen chloride, a colourless gas, and HCl(aq) is hydrochloric acid. HCl without (g) or (aq) does not, strictly speaking, refer to either of the two substances.
BEHAVIOUR OF BRØNSTED-LOWRY BASES IN WATER
When a base is added to water, it accepts a proton from the water to form a hydroxide ion. For example, when sodium oxide, Na2O, is added to water, it is ionized and the oxide ion O2- accepts a proton from a water molecule to form a hydroxide ion:
O2-(s) + H2O(l) → OH-(aq) + OH-(aq)
The equation has been written with two separate hydroxide ions to emphasize that the oxide ion becomes part of a hydroxide ion when it accepts a proton from a water molecule. As another example, ammonia, a colourless gas, dissolves in water to form an alkaline solution:
NH3(g) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
This time, the whole ammonia molecule accepts a proton from a water molecule to form the ammonium ion.
AMPHOTERIC NATURE OF WATER
In water, there is always a very small proportion of OH-(aq) and of H+(aq). This is the result of an acid-base reaction between two water molecules:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH-(aq)
One water molecule donates a proton and the other accepts a proton. This demonstrates that water can behave both as an acid and as a base, so it is an amphoteric compound.
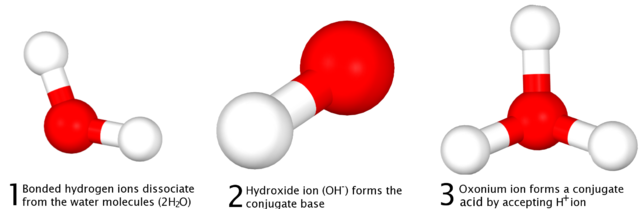
This acid-base reaction can be written in a simpler form as the dissociation of water:
H2O(l) ⇌ H+(aq) + OH-(aq)
DISSOCIATION IN BRØNSTED-LOWRY ACIDS
When a Brønsted-Lowry acid is put into water, a chemical reaction called dissociation or ionisation takes place. A covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission to leave a proton and a negative ion. So, when the gas hydrogen chloride dissolves in water, it dissociates to form a proton or hydrogen ion, and a chloride on:
HCI(g) → H+(aq) + Cl-(aq)
The arrow in the equation indicates that, in water, every hydrogen chloride molecule dissociates.
In water, sulfuric acid dissociates to form two hydrogen ions per sulfuric acid molecule:
H2SO4(aq) → 2H+(aq) + SO4(aq)2-
The arrow in the equation indicates that, in water, every sulfuric acid molecule dissociates, but in reality many of the sulfuric acid molecules only dissociate to form the hydrogensulphate ion, HSO4-(aq).
Conjugate bases
During the dissociation of hydrogen chloride, all the molecules dissociate to form aqueous hydrogen ions and chloride ions. The chloride ion is known as the conjugate base of hydrochloric acid. This does not mean that the chloride ion is a base, but that in theory it could accept a proton. That is, it could behave as a base. The conjugate base of any acid is the anion formed after dissociation.
Note that the conjugate base of sulfuric acid is the hydrogensulfate ion HSO, rather than the sulfate ion, HSO4(aq)-.
A table showing five acids and their dissociations
| Acid | Conjugate base | |||
|---|---|---|---|---|
| HCl(aq) | → | H+(aq) | + | Cl-(aq) |
| HNO3(aq) | → | H+(aq) | + | NO3- |
| H2SO4(aq) | → | H+(aq) | + | HSO4(aq)- |
| HBr(aq) | → | H+(aq) | + | Br-(aq) |
| HI(aq) | → | H+(aq) | + | I-(aq) |
Conjugate acids
Just as a Brønsted-Lowry acid has a conjugate base, so a Brønsted-Lowry base has a conjugate acid, which is the particle formed once the base has accepted a proton. For example, the conjugate acid of ammonia, NH3, is the ammonium ion, NH4, and the conjugate acid of the hydroxide ion, OH-, is the water molecule, H2O.
Strong acids
The table above shows how five common covalently bonded acids dissociate when in water. In each case, almost all the molecules of the acid dissociate to form ions. In fact, we assume that all of the molecules dissociate, and represent the dissociation by an arrow in the equation. Such acids are called strong acids, a term meaning that almost all of the acid's molecules dissociate when it is dissolved in water. Do not confuse a strong acid with a concentrated acid. A strong acid is fully dissociated in water, but a concentrated acid has a high number of moles of acid in 1 dm3 of solution.
Weak acids
Other acids, such as ethanoic acid, dissolve in water, but do not fully dissociate. Only a very small percentage of their molecules may be dissociated at any one time. They are known as weak acids. For example, in a solution containing 0.1 mol dm-3 of ethanoic acid, only 1.3 per cent of the molecules dissociate. Ethanoic acid is therefore a weak acid. Do not confuse a weak acid with a dilute acid. The term weak acid means that only a small proportion of molecules dissociate when the acid is dissolved in water:
CH3COOH(aq) ⇌ CH3COO-(aq) + H-(aq)
Note that the dissociation equation includes the symbol for a reversible reaction instead of an arrow. This indicates that the dissociation of ethanoic acid and the association of the ethanoate ion and the hydrogen ion occur at the same time.
Once ethanoic acid has dissolved in water, the rate of the dissociation reaction soon reaches that of the association reaction, so that there is no net change in the concentrations of the undissociated ethanoic acid, the ethanoate ion and the hydrogen ion. We say that the reaction reaches a state of dynamic equilibrium, in which the concentration of each species remains the same.
I will stop here for now and continue in the next post where I will discuss the acid dissociation constant and how to measure the pH of a solution, and the carboxylic acids.
Thanks for coming.
REFERENCES
https://www.atlantis-press.com/journals/efood/125918568/view
https://www.news-medical.net/health/pH-in-the-Human-Body.aspx
https://www.hindawi.com/journals/jeph/2012/727630/
https://en.wikipedia.org/wiki/Acid
https://www.britannica.com/science/Bronsted-Lowry-theory
https://en.wikipedia.org/wiki/Acid%E2%80%93base_reaction
https://www.britannica.com/science/acid-base-reaction
https://www.topperlearning.com/answer/explain-the-amphoteric-nature-of-water/4ubr799uu
https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Amphoteric
https://www.britannica.com/science/acid-base-reaction/Dissociation-of-molecular-acids-in-water
https://en.wikipedia.org/wiki/Br%C3%B8nsted%E2%80%93Lowry_acid%E2%80%93base_theory
http://guweb2.gonzaga.edu/faculty/cronk/CHEM101pub/bronsted.html
https://opentextbc.ca/introductorychemistry/chapter/bronsted-lowry-acids-and-bases-2/
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules
https://www.thoughtco.com/definition-of-conjugate-acid-605846
https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/acids-and-bases/a/bronsted-lowry-acid-base-theory
https://socratic.org/chemistry/acids-and-bases/conjugate-acids-and-conjugate-bases
https://en.wikipedia.org/wiki/Conjugate_acid
https://opentextbc.ca/introductorychemistry/chapter/strong-and-weak-acids-and-bases-and-their-salts-2/
https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/acids-and-bases/e/identifying-weak-acids-and-strong-acids-exercise
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules
https://www.chemguide.co.uk/physical/acidbaseeqia/acids.html
!discovery 30
Thank you for the constant support, @delilhavores and @discovery-it. I sincerely appreciate your kind gesture.
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
You have done justice to the chemistry of acid. It's a substance required in a moderate amount at times for preservative purpose. And it's over good that you differentiate between a weak acid and a dilute acid, at times students mix things up there. Your choice of words simplify chemistry for beginners. Well done @empresstemah.
You are certainly right about acid, @steepup. Thanks for coming by and thank you for the nice comment too.