All About Gases As Being Taught In the Classroom #5
Hello, dear readers, students and colleagues. In my last post on All About Gases As Being Taught In the Classroom #4, I explained the vapour pressure , the unusual behaviour of water, aqueous solutions, concentration and preparation of solutions of known concentration. But, in today's post which will be the final post on the series, I will delve into what titration entails and give some examples. I will then go ahead to discuss the entropy in gas, liquid and solid.
TITRATIONS
A titration is an experimental technique that uses the known volumes of two solutions to determine the amounts of substances that are reacting very accurately. This technique is often referred to as volumetric analysis.
In a titration one of the solutions is of known concentration. If we know the balanced equation for a reaction, then we know how many moles of each reactant will react. Using this information we can calculate the concentration of the other solution.

Acids are neutralized by bases. If we wish to discover the concentration of a dilute hydrochloric acid solution we can perform an acid-base titration. Sodium hydroxide of known concentration is used as the base to neutralize exactly the hydrochloric acid. The reaction is followed using an acid-base indicator, which changes colour at the neutralization point or end point or equivalence point. The choice of indicator depends on the type of acid and base used.
EXAMPLE 1.
What is the concentration of a dilute solution of hydrochloric acid if 26.1 cm³ is exactly neutralized by 25.0 cm³ of 0.100 mol dm-3 sodium hydroxide?
ANSWER
Step 1. Write the balanced equation:
NaOH(aq) + HCI(aq) NaCl(aq) + H2O(aq)
Step 2. Convert the equation into amounts:
1 mol NaOH + 1 mol HCl → 1 mol NaCl + 1 mol H2O
Step 3. Work out the amount of sodium hydroxide being used:
Number of moles NaOH in 1000 cm³ = 0.100 mol
So number of moles NaOH in 25.0 cm³ = 0.100 × 25/1000 = 0.002 50 mol
Step 4. Scale the amounts in the equation:
1 mol NaOH reacts with 1 mol HCI
0.002 50 mol NaOH reacts with 0.00250 mol HCI
So there are 0.00250 mol HCI in 26.1 cm³ Solution.
Step 5. Scale the amount in the known volume to 1 dm³:
0.002 50 mol HCI in 26.1 cm³ solution
In 1 dm³ (1000 cm³) moles HCI = 0.002 50 × 1000/26.1
Therefore concentration of HCI = 0.095 8 mol dm-3
EXAMPLE 2.
Clover leaves contain ethanedioic acid, (COOH)2. 1.01g of clover leaves are crushed and the ethanedioic acid is extracted using a small volume of distilled water. This solution is exactly neutralized by 17.2 cm³ of 0.200 mol NaOH. What is the mass of ethanedioic acid in this sample of leaves?
ANSWER
Step 1. Write the balanced equation:
(COOH)2 (aq) + 2NaOH(aq) → (COO-Na+)2 (aq) + 2H2O(l)
Step 2. Convert the equation into amounts:
1 mol (COOH)2 + 2 mol NaOH — 1 mol (COO- Na+)2(aq) + 2 mol H2O(l)
Step 3. Work out the amount of sodium hydroxide being used:
Number of moles NaOH in 1000 cm³ = 0.200 mol
So number of moles NaOH in 17.2 cm³ = 0.200 × 17.2/1000 = 0.003 44 mol
Step 4. Scale the amounts in the equation:
1 mol (COOH)2 reacts with 2 mol NaOH
0.00172 mol (COOH) 2 reacts with 0.003 44 mol NaOH
So there are 0.00172 mol (COOH) 2 in the clover leaf solution.
Step 5. Convert the amount (moles) into a mass:
Mass in grams = amount in moles × mass of 1 mole
Mass of 1 mol of (COOH)2 = (12 + (16 × 2) +1 g) × 2 = 90g
So the mass of ethanedioic acid in 1.01 g clover leaves = 0.001 72 mol × 90 g
= 0.155 g (COOH)2
ENTROPY
Entropy is a measure of the disorder of a system. Think of your bedroom – when it is tidy everything has a place and there is a high degree of order. We could say that the entropy of your room is low. However, it is all too easy for the entropy to rise as things are moved out of place and it becomes untidy.
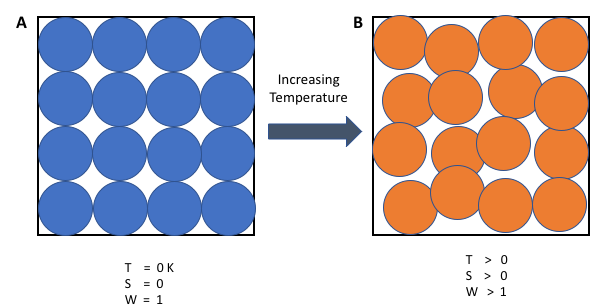
When particles are in a solid they cannot move out of position, they can only vibrate. In the solid state there is usually a high degree of order and a limited number of ways to arrange the particles, so the entropy of a solid is usually low. The liquid state tends to have a higher entropy than the solid state, because the particles can move about, so many more arrangements are possible. A gas has widely spaced particles that move very rapidly in all directions. All gases have high entropies.
The entropies of different substances can be determined for a particular temperature. Entropy is given the symbol S and is measured in joules per Kelvin per mole (JK-1mol-1). In a table that's showing the entropy values for some substances at 298 K and 101 kPa, you will observe that water vapour has a much higher entropy than water liquid. This is to be expected, since water molecules are more spread out in the vapour and more arrangements are possible. Diamond has a lower entropy than graphite, which means that diamond has a more ordered structure. Neon and helium are both gases and so have relatively high entropies.
However, neon has heavier atoms. This tends to bring its energy levels closer together, which in fact provides more possibilities of arranging quanta of energy.
Entropy, then, is not just about the number of possible ways of arranging particles. It is also about how the energies of the particles are arranged in the energy levels, an aspect of entropy that I will continue later at another time.
At zero Kelvin, perfect crystals have zero entropy. This is the third law of thermodynamics. Perfect crystals have only one arrangement of particles – they are perfectly ordered in all directions. At 0 K, molecular motion virtually stops, so there is only one arrangement of particles and energy, hence zero entropy.
SUMMARY
After reading extensively through my series on GASES, you should know and understand the following points and be able to use the equations.
- Equal volumes of gases contain equal numbers of molecules at the same temperature and pressure (Avogadro’s law).
- 1 mole of any gas at standard temperature and pressure occupies 22.4 dm³. Under room conditions the molar gas volume is 24 dm³,
- Boyle’s law relates the pressure and volume of a fixed amount of gas and is expressed mathematically as: P ∝ 1/V, where p =pressure and V= volume.
- Charles’ law relates volume and temperature of a gas and is expressed mathematically as: V ∝ T, where T = absolute temperature in kelvin.
- The ideal gas equation relates pressure, volume and temperature for a fixed amount of gas: pV = nRT.
- The kinetic-molecular model explains the behaviour of ideal gases by making a number of assumptions, such as zero intermolecular forces and that the volume of particles is negligible.
- Real gases approach ideal behaviour when at a high temperature or low pressure.
- The kinetic-molecular model can be extended to include solids and liquids.
- Vapour pressure is the pressure above a liquid when it is in equilibrium with its vapour. Vapour pressure increases with temperature.
- Hydrogen bonding is a strong intermolecular force between hydrogen bonded to an F, N or O atom and the lone pair of another F, N or O atom. It accounts for the anomalous properties of water.
- The concentration of a solute in a solution is measured in mol dm-3 or g dm-3.
- Titrations are a valuable way to analyse amounts in solutions.
- Entropy is a measure of the disorder of a system. Solids usually have low entropies, whereas gases have high entropies.
Thanks for reading
##REFERENCES
https://www.britannica.com/science/titration
https://courses.lumenlearning.com/introchem/chapter/acid-base-titrations/
https://en.wikipedia.org/wiki/Titration
https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Demos%2C_Techniques%2C_and_Experiments/General_Lab_Techniques/Titration
https://www.britannica.com/science/entropy-physics
https://en.wikipedia.org/wiki/Entropy
Nice post! Thank you for sharing :)
!discovery 30
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.