All About Gases As Being Taught in the Classroom #4
Hello, dear readers, students and colleagues. Today's post is a continuation from where I stopped on All about gases as being taught in the classroom #3. And the topics are vapour pressure, aqueous solutions and concentration. So, what do you understand about vapour pressure ……?
VAPOUR PRESSURE
Although a liquid changes into a gas at its boiling point, we know that a liquid can change into a gas at temperatures below the boiling point. If a glass of water is left on a windowsill for a week, some of it changes into a gas and the volume of water decreases. The process is evaporation. At any temperature, the particles in a liquid have a range of kinetic energies. Some particles have enough energy to break free of the forces that hold them in the liquid state and they evaporate. Since it is the particles with the most kinetic energy that evaporate, the particles with lower kinetic energies are left in the liquid, so the average kinetic energy of the liquid drops and the temperature of the liquid fall.

On a windy day in winter, weather forecasters talk about the wind chill factor. This is because air moving over the skin causes water to evaporate from the skin faster, so the apparent temperature we feel is much colder than the air temperature.
If the liquid is in a closed vessel, then the rate at which the particles escape from the liquid surface equals the rate at which the particles rejoin the liquid. When the rate of evaporation is the same as the rate of condensation, we say that the liquid is in equilibrium with its vapour (or gas). This is a dynamic equilibrium. The pressure caused by the vapour above the liquid at equilibrium is called its vapour pressure.
The vapour pressure is independent of the volume of the closed vessel, the volume of the liquid and the volume of the vapour. As the temperature increases, so does the average kinetic energy of the particles in the liquid. This means that more particles have sufficient energy to escape into the gas and so the vapour pressure increases. When the vapour pressure of a liquid is the same as the external pressure above its surface, then the liquid boils. For example, water boils when its vapour pressure equals atmospheric pressure. This is why water boils at 70 °C on the top of Mount Everest, where the atmospheric pressure is very much less than it is at sea level.
Sublimation and freeze-dried coffee
When a solid changes straight to a gas without passing through the liquid state, the change of state is known as sublimation. The reason that some solids can do this is that they possess relatively high vapour pressures. Iodine and carbon dioxide are two examples of solids with high vapour pressures, so at atmospheric pressure their solid forms sublime.
While we expect ice to melt rather than sublime, it can change straight to water vapour: you may have noticed that frozen puddles gradually shrink in freezing weather. This phenomenon is used in the production of instant coffee. The coffee is brewed and then frozen in a container from which air is removed by a vacuum pump. Lowering the pressure causes the ice in the frozen brewed coffee to sublime. When nearly all the ice has been removed, the coffee is said to be freeze-dried and is ready for packaging. This method of removing water leaves the flavour molecules intact, and so freeze-dried instant coffee has a much better flavour than coffee that is dried by slow heating.

THE UNUSUAL BEHAVIOUR OF WATER – HYDROGEN BONDING
Water is a remarkable liquid. Indeed, if you compare its boiling point to those of the hydrides of other Group 6 elements you could be forgiven for thinking that it should not really be a liquid at all at room temperature.
Water’s surprisingly high boiling point and melting point results from a special intermolecular bonding known as hydrogen bonding. Hydrogen bonds form when hydrogen is covalently bonded to the very electronegative elements oxygen, fluorine and nitrogen.
In water, the hydrogen-oxygen covalent bond is very polar. Hydrogen has only one electron, so when the oxygen atom in water pulls the bonded pair of electrons towards itself, it leaves the hydrogen atom’s nucleus of a single proton more exposed. The resulting δ+ on the hydrogen atom is attracted to the lone pair (δ-) of the oxygen atom to form a very strong intermolecular attraction.
Hydrogen bonds are very strong compared to other intermolecular forces. This means that for water to boil these strong hydrogen bonds have to be broken before the molecules can escape into the vapour, which leads to the relatively high boiling point of 100 °C at sea level (101 kPa).
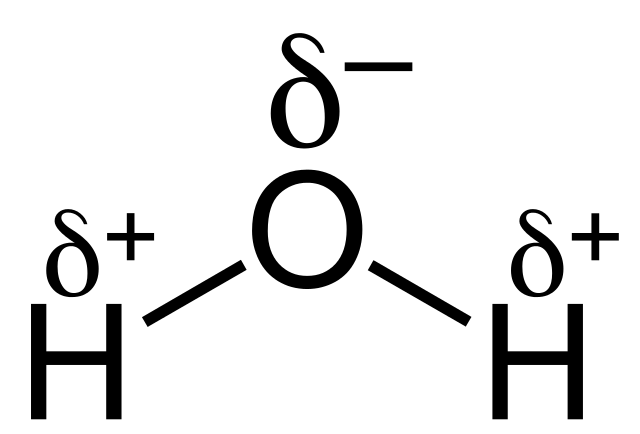
Hydrogen bonding also explains why water expands on freezing. As water cools, the molecules come closer together and so the density increases until, at 4 °C, the density is at a maximum. Below this temperature, the water molecules start to move apart because of the increasing number of hydrogen bonds they form. When water freezes, the water molecules are held apart by hydrogen bonds in a three-dimensional lattice. Thus the ice is less dense than water, which explains why ice is one of the very few solids to float on its liquid.
AQUEOUS SOLUTIONS AND CONCENTRATION
Aqueous solutions have water as the solvent. When we write equations and we want to show that a reactant is dissolved in water, we write the state symbol (aq). So a dilute solution of sulfuric acid is written: H2SO4(aq).
Water covers most of the surface of our planet, and many reactions take place in aqueous solution. Nearly all the chemical reactions of our bodies occur in the aqueous environment of our cells.
CONCENTRATION
Concentration can be expressed in terms of the mass of a substance in a certain volume of solution. The units often used are grams per cubic decimetre or g dm-3. So if 40 grams of sodium hydroxide are present in one cubic decimetre the concentration is 40 g dm-3. Chemists, however, are usually interested in the amount of a substance present.
When I considered gases at the beginning of this chapter, it was not long before we came across the mole. It is the same with solutions. We need to know how many moles of solute are present in a particular volume of solution, because this tells us the number of particles that are there.
The concentration of a solute in a solution is measured in moles per cubic decimetre – mol dm-3.
So, here are two worked examples to understand more about concentration of aqueous solutions.
EXAMPLE 1.
Work out how many moles of solute are dissolved in these solutions:
a. 250 cm³ of 1.0 mol dm-3 NaOH(aq);
b. 20 cm³ of 0.50 mol dm-3 HCl(aq)
ANSWER
a. 1 dm³ = 1000 cm³
Number of moles NaOH in 1000 cm³ = 1 mol
So number of moles NaOH in 250 cm³ = 1 × 250/1000 = 0.25 mol
b. Number of moles HCl in 1000cm³ = 0.50mol
So number of moles HCl in 20 cm³ = 0.50 × 20/1000 = 0.010 mol
EXAMPLE 2.
What is the concentration (in mol dm-3) of the following aqueous solutions?
a. 0.25 mol HCl dissolved in 50 cm³;
b. 5.85 g of NaCl dissolved in 250 cm³.
ANSWER
a. There are 0.25 mol HCI in 50 cm³
So in 1000 cm³, the number of moles = 0.25 × 1000/50 = 5.0 mol
Concentration of HCl in solution = 5.0 mol dm-3
b. Moles of NaCl in 5.85 g
mass in grams/mass of one mole (in grams) = 5.85g/58.5g = 0.100 mol
There are 0.100 mol NaCl in 250 cm³
So in 1000 cm³, the number of moles = 0.100 × 1000/250 = 0.400 mol
Concentration of NaCl solution = 0.400 mol dm-3
PREPARING SOLUTIONS OF KNOWN CONCENTRATION
Chemists often need to make up very accurately a solution of known concentration. To do this a volumetric flask is used. On its long neck is etched a line that indicates a precise volume. The required amount of substance to be dissolved to make up the solution is weighed accurately on a balance. It is then dissolved in a small volume of distilled water in a beaker and the solution is poured into the volumetric flask. Distilled water is then used to rinse any remaining solution from the beaker into the flask. More distilled water is added until the meniscus in the neck of the flask just lies on the etched line.
Below are are some examples on how to prepare solutions of known concentration.
EXAMPLE
What mass of sodium hydroxide is required to make up 250.0 cm³ of a 0.50 mol dm-3 sodium hydroxide solution?
[Ar: Na = 23, O = 16, H = 1].
ANSWER
Step 1. Work out the amount (moles) required:
1 dm³ = 1000 cm³
Number of moles NaOH required in 1000 cm³ = 0.50 mol
So number of moles NaOH required in 250 cm³ = 0.50 × 250/1000 = 0.125 mol
Step 2. Convert amount (moles) of NaOH into a mass:
Mass in grams = amount in moles × mass of 1 mole
Mass of 1 mol of NaOH = 23 + 16 + 1g = 40 g
So mass of sodium hydroxide = 0.125 mol × 40 g = 5.0 g
Therefore 5.0 g must be dissolved in 250 cm³ to make a 0.5 mol dm-3 solution.
Finally, remember that the concentration of a solution is expressed per dm³ of solution and not per dm³ of solvent. If one mole of solid is added to 1 dm³ of water the volume of the solution will probably be slightly greater than 1 dm³, so the concentration will not be exactly 1 mol dm-3.
In the next post, which is also going to be my last post on this series, I will like to discuss titration and entropy.
Thanks for coming.
REFERENCES
https://www.britannica.com/science/vapor-pressure
https://en.wikipedia.org/wiki/Vapor_pressure
https://en.wikipedia.org/wiki/Freeze-drying
https://www.buencafe.com/en/blogs/freeze-dried-coffee/
https://www.newfoodmagazine.com/article/16968/freeze-drying-in-the-coffee-industry/
https://link.springer.com/article/10.1007/s12043-008-0191-0
https://www.khanacademy.org/science/biology/water-acids-and-bases/hydrogen-bonding-in-water/a/hydrogen-bonding-in-water
http://www1.lsbu.ac.uk/water/water_hydrogen_bonding.html
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_
http://www.lsbu.ac.uk/water/anmlies.html
https://courses.lumenlearning.com/trident-boundless-chemistry/chapter/solution-concentration/
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_
https://www.chem.ucla.edu/~gchemlab/soln_conc_web.htm
https://www.thoughtco.com/how-to-prepare-a-solution-606091
http://faculty.sites.uci.edu/chem1l/files/2013/11/RDGsolnprep.pdf
https://chem.libretexts.org/Courses/Prince_Georges_Community_College/General_Chemistry_for_Engineering/Unit_4%3A_Nomenclature_and_Reactions/Chapter_12%3A_Aqueous_Reactions/Chapter_12.1%3A_Preparing_Solutions
Congratulations @empressteemah! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!
@tipu curate
Upvoted 👌 (Mana: 8/12)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.