All About Gases As Being Taught In the Classroom #2
Hello, dear readers. This is the continuation of my post on ALL ABOUT GASES AS BEING TAUGHT IN THE CLASSROOM. I'll start today's class by explaining all about the gas laws. So, kindly follow me as I go on…
THE GAS LAWS
Gas is in some ways the simplest of the three states of matter. Through doing countless experiments, scientists have investigated the physical properties of different gases. They have found that, with minor variations, all gases behave in a similar way under room conditions and that there are definite relationships between pressure, volume, temperature and amount. These relationships have been defined in what we call the gas laws.

THE RELATIONSHIP BETWEEN PRESSURE AND VOLUME: BOYLE’S LAW
If you have read the introduction of my post on The Birth of Elements and the Discovery of the Nucleus, you will know that Robert Boyle had a deep influence on modern chemistry in urging chemists to do experiments to find elements. He also conducted his own experiments on gases.
In 1662 he carried out a series of experiments using a glass tube 5 metres high, shaped like a letter J and closed at the short end. He trapped an amount of air in the short end of the J tube by pouring mercury into the other end. He found that the more mercury he added, the smaller the volume of air became. He had thus discovered that the volume of air depended on the pressure of the mercury upon it, a finding he summarised in what we now know as Boyle’s law:
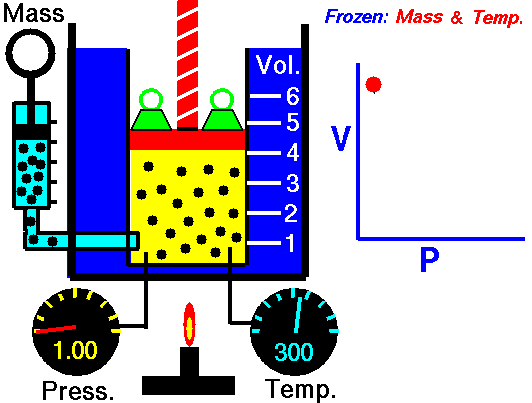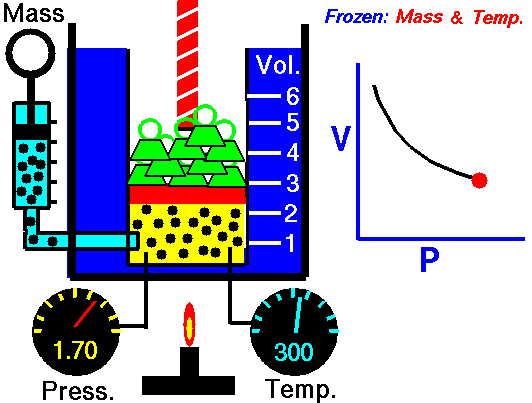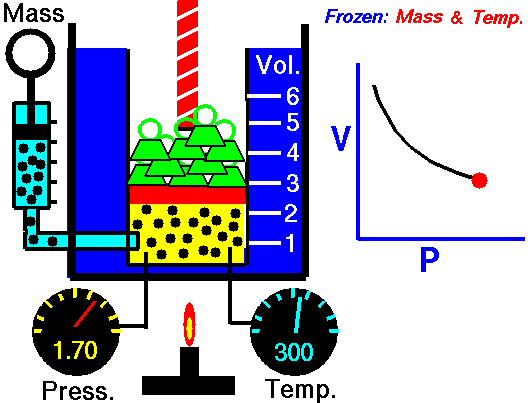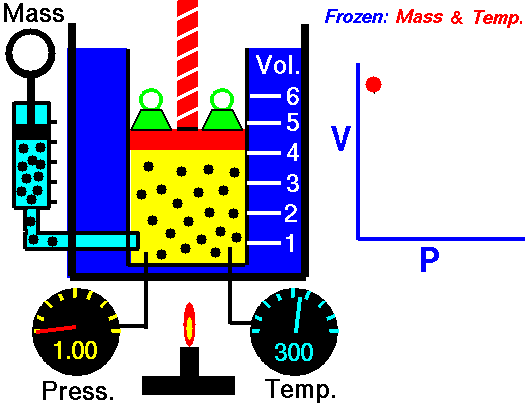
The volume of a fixed amount of gas is inversely proportional to its pressure at constant temperature.
We can express this mathematically as:
P ∞ 1/V or pV = a constant
We can show this graphically by plotting pressure against 1/volume as the figure shown. So, if we double the pressure on a gas the volume will halve, and if the volume increases four times then the pressure on the gas must have decreased to a quarter, provided the temperature stays constant.
Pressure p multiplied by volume V is always equal to the same constant for a fixed amount of gas at the same temperature. So we can write:
p1V1= k = p2V2 or p1V1 = p2V2
Where p1 and V1 are one set of conditions, and p2 and V2 are another set. We can use this equation, which comes from Boyle’s law, to work out the effect of changing the pressure on a particular volume of a gas. It can also be used to find the effect on the pressure exerted by a gas of changing the volume of that gas.
As I promised in my first post, that I would be explaining this concept as if it were to be taught in a classroom. So, I will be sharing as many examples as possible here.
EXAMPLE 1.
A 400 cm3 mixture of petrol vapour and air is taken into the cylinder of a car engine at 200°C and a pressure of 100 kPa. The piston compresses this gaseous mixture to 50 cm3. What is the pressure of the compressed gas if the temperature does not change?
ANSWER
Initial conditions: P1 = 100 kPa and V2 = 400 cm3
Final conditions: P2 = ? and V2 = 50 cm3
Since, p1V1 = p2V2
100 x 400 = p2 x 50
p2 = 800 kPa
Breathing and Boyle’s law
When we are at rest we breathe in and out about twelve times a minute. As we breathe in, the volume of our chest cavity expands, increasing the volume of our lungs. This causes the pressure of air inside the lungs to decrease, since the pressure of the air outside our bodies is now greater than that inside, air flows in. The reverse happens when we breathe out: the chest volume reduces, which increases the pressure of air in the lungs. The air is now at a higher pressure than that outside, so air flows out.
THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE: CHARLES’ LAW
During the 18th century, several experimenters, including Boyle, noticed that temperature had an effect on the volume of a gas. In about 1800, two French scientists carried out experiments on the relationship between volume and temperature, as a spin-off from their ballooning activities. Jacques Charles was the first and Gay-Lussac, working independently, was the second.
Both workers discovered that, when a fixed amount of gas was kept at constant pressure, the volume varied in proportion to the temperature. Gay-Lussac also noticed that if the volume of gas at 0°C was taken, then for every 1 °C drop in temperature the volume decreased by 1/273 under the same conditions of pressure and amount. This suggested that at -273 °C there would be a zero volume of gas.
It was Lord Kelvin, a British scientist, who realised the significance of this 50 years later. He called -273 °C the absolute zero temperature, below which it was impossible to go. This gave rise to a new temperature scale – the absolute temperature scale. Absolute zero has a value of 0K (zero kelvin) and a 1 K rise in temperature is the same as a 1°C rise in temperature. This means that at 0°C the temperature on the Kelvin scale is 273 K.
The relationship between volume and temperature is called Charles’ law.
The volume of a fixed amount of gas is directly proportional to its absolute temperature at constant pressure.
This can be expressed mathematically:
V∞ T or V = kT
where k is a constant and T is absolute temperature. Note that k is not the same as the constant in Boyle’s law.
For a fixed amount of gas at the same pressure, the same constant will apply, so we can write:
V1/Т1 = k = V2/T2 so V1/T1 = V2/T2
EXAMPLE 1.
A balloon is filled with 1250 cm3 of helium gas at 25.0 C. Overnight the temperature cools to 10.0 °C. What is the new volume of the balloon, assuming the pressure is constant?
ANSWER
The temperatures must first be converted into absolute temperatures. To do this, 273 is added to the temperature in Celsius so:
25°C = 273 + 25 = 298 K, and 10 °C = 273 + 10 = 283 K
Initial conditions: V1 = 1250 cm3 and T1 = 298 K
Final conditions: V2 = ? cm3 and T2 = 283 K
Since: V1/T1 = V2/T2
1250/298 = V2/283
1250 x 283/298
V2 = 1190 cm3 (to three significant figures)
THE IDEAL GAS EQUATION
Another way of expressing Avogadro’s law is: The volume of a gas is directly proportional to its amount at constant temperature and pressure. This can be expressed mathematically as:
V ∞ n
where n = amount in moles.
So the volume of a gas is related to three other properties: amount, temperature and pressure.
Avogadro’s law V ∞ n (constant p and T)
Boyle’s law V ∞ 1/p (constant n and T)
Charles’ law V ∞ T (constant n and p)
Combining these: V ∞ nT/p or V = RnT/p
Where R is a constant called the gas constant. Rearranging this equation gives the ideal gas equation:
pV = nRT
When p is measured in pascals (Pa), V in cubic metres (m3), n in moles (mol) and T in kelvins (K), R is 8.31 JK-1 mol-1. These units are internationally agreed and are called SI units, after the French words Système Internationale.
Gases that obey the ideal gas equation exactly are called ideal gases. In reality, no gas is an ideal gas, but the variations from ideal behaviour are quite small over wide ranges of temperature and pressure. This enables us to use the ideal gas equation as a very useful tool to relate the four properties of volume, temperature, pressure and amount.
EXAMPLE 3.
At 90.0 °C how many moles of nitrogen are present in a flask of volume 750.0 cm3 at 100 kPa pressure?
ANSWER
First convert the units into SI units.
The volume in cm3 must be converted into m3. There are 1003 cm3 in 1 m3
(i.e. 106 cm3), so:
V = 750.0 × 10-6 = 7.50 × 104 m3
p = 100 × 103 Pa
T = 273 + 90.0 = 363 K
Then substitute into the ideal gas equation:
pV = nRT so n = pV/RT
n = 100 × 103 × 7.50 × 10-4/8.31 × 363
n = 0.0249 mol
USING THE IDEAL GAS EQUATION TO CALCULATE RELATIVE MOLECULAR MASS
Nowadays, mass spectrometers are used to measure relative molecular mass Mr accurately. However, when this instrument is not available the Mr of gases and volatile liquids can be calculated using the ideal gas equation:
pV = mRT/Mr
One way of doing this is to use a gas syringe. If the substance is a gas, it can be passed into a syringe of known mass and the syringe reweighed to give the mass of gas. The volume of gas is read from the syringe and the temperature and pressure are measured. Then these values are simply inserted into the ideal gas equation. Remember that amount in moles:
n = mass in grams/mass of one mole (in grams)
Also, Mr is numerically equal to the mass of one mole.
EXAMPLE 4.
The gas propane contains hydrogen and carbon only. When 100.0 cm3 of it is passed into a syringe, the mass is found to be 0.178 g. The pressure and temperature are 298 K and 100 kPa, respectively. Calculate the Mr.
ANSWER
The measurements are converted into SI units, with the exception of the mass, which is left as grams. (Although, the SI unit for mass is the kilogram.)
P = 100 × 103 kPa, V = 100 × 10-6 m3, m = 0.178 g, Mr= ?, R = 8.31 JK-1 mol-1 and T = 298 K.
Insert these values into the ideal gas equation:
pV = mRT/Mr
100 × 103 × 100 × 10-6 = 0.178/Mr × 8.31 × 298
Rearranging: Mr = (0.178 × 8.31 × 298) ÷ (100 × 103 × 100 × 10-6
Mr = 44.1

The same method is used to determine the Mr of a volatile liquid, only this time the end of the syringe is sealed with a self-sealing rubber cap through which a known mass of the liquid under investigation is injected. Since the liquid is volatile it has a low boiling point, and if the syringe is heated to above the boiling point the volume of the gas produced can be measured on the syringe. In practice, there is usually a small volume of air in the syringe. So long as this volume is known at the temperature of the experiment, It can be subtracted from the volume of the vaporised sample.
Till next time when I'll be discussing the kinetic molecular model for how gases behave and how particles are arranged in liquids and solids.
Thank you for reading.
REFERENCE
https://en.wikipedia.org/wiki/Gas_laws
https://www.britannica.com/science/gas-laws
http://chemistry.bd.psu.edu/jircitano/gases.html
https://courses.lumenlearning.com/introchem/chapter/boyles-law-volume-and-pressure/
https://en.wikipedia.org/wiki/Boyle%27s_law
https://www.britannica.com/science/Boyles-law
https://www.cliffsnotes.com/study-guides/anatomy-and-physiology/the-respiratory-system/mechanics-of-breathing
https://courses.lumenlearning.com/boundless-biology/chapter/breathing/
https://www.ck12.org/c/physical-science/boyles-law/rwa/Breathing-Muscles/
https://www.britannica.com/science/Charless-law
https://ch301.cm.utexas.edu/section2.php?target=gases/gas-laws/charles-law.html
https://en.wikipedia.org/wiki/Charles%27s_law
https://en.wikipedia.org/wiki/Ideal_gas_law
https://www.chemguide.co.uk/physical/kt/idealgases.html
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Gases/Gas_Laws/The_Ideal_Gas_Law
https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/14.9/primary/lesson/calculating-the-molar-mass-of-a-gas-chem
http://www.docbrown.info/page03/3_52gaslaws2.htm
https://edu.rsc.org/resources/determining-relative-molecular-masses-by-weighing-gases/832.article
https://study.com/academy/lesson/using-the-ideal-gas-law-to-find-the-molar-mass-of-a-gas.html
Without being told, one would know that you (the author) are a teacher. This is quite simplified and clear. I hope you can get some of your students to sign up and even answer questions below your posts.
Thanks for coming, @hadji.
Yes, you are right. Once a teacher, always a teacher. I have a passion for teaching and I really hope some of my students can sign up and answer many of my questions. But, I know that one day, even if not my students, some students would immensely benefit from all these posts.
Thank you again for the compliment.
you are welcome
As a civil engineer, chemistry and study of gases are not really my field, however, I remember some of the laws you explain, I studied some of that in my first semesters. I'll never forget Serway-Jewett Physics book.
Thank you @acont. The gas laws cut across both chemistry and phyiscs. It's a very interesting topic that I did both in two field.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.