ORDINARY MATTER #2
Greetings to you all. Still on the physics of ordinary matter and changing the state of a substance. I will start today’s discussion by explaining the dependence of melting and boiling temperatures on pressure and then go on to discuss more on atoms and molecules.
DEPENDENCE OF MELTING AND BOILING TEMPERATURES ON PRESSURE
So far, we have assumed that the water is at standard pressure. In fact, the temperatures of melting and boiling alter if there is a change in pressure of the surroundings. The change in pressure needs to be large for us to notice the effect, but we do commonly meet it.
If we increase the pressure on a block of ice, the freezing temperature of the ice decreases. This is what happens when we ice-skate. The sharp blades of the skate apply a large force to the ice over a small area. There is a very large pressure under the blades which helps the ice to melt under the blades. The water effectively lubricates the skating surface.

The temperature of the ice and the width of the blades both need to be carefully controlled. If the skates were designed with very broad blades, they would not apply enough pressure. Even using narrow blades, it is not possible to skate on ice that is at too low a temperature.
The temperature of vaporisation of water is lowered by a decrease in pressure, so that water boils at a lower temperature on a mountain top, where the atmospheric pressure is lower, than at sea level. High up a mountain is not the best place to make a good cup of tea. Though the boiling temperature is not lowered much, the drop is enough to reduce the amount of flavour extracted from the tea leaves! You may also notice this on a plane flight.
Sublimation
Some substances can change directly from the solid state to the vapour state. This process is called sublimation. The temperature and pressure may need adjusting for this to happen. For example, solid carbon dioxide (dry ice) remains solid up to -78 °C, then it changes directly into the gas. Dry ice is used in research laboratories for keeping equipment and samples cold.
It is also used in the theatre for creating an artificial fog – the evaporating carbon dioxide molecules cool the air and water in the air condenses out as droplets.
ATOMS AND MOLECULES
Solids can form many different structures at the microscopic level. In all cases, the solids consist of atoms held closely together, though the arrangements of these atoms can differ considerably. Here I am going to look at some of these patterns, especially those that are regular. For this, I will use a very simple model in which the atoms are considered to be identical hard spheres. Bringing the atoms close together is similar to packing marbles or polystyrene spheres next to each other.
If the marbles or polystyrene spheres are pushed together, they resist compression once their outer surfaces touch. Since marbles are hard, they resist compression more than the polystyrene spheres. In both cases, there is a force opposing the movement.
In a similar way, there will be a force of repulsion when two atoms are brought together. Here it is due to the outer electrons repelling each other as the atoms become close. The force of repulsion, shown by the red curve in the figure, gets much larger as the separation between atoms is reduced.
Hence we will have no difficulty in separating the marbles or polystyrene spheres – there are no attractive forces to overcome. However, atoms do resist separation, because there is an attractive force. It has a negative value. But this attractive force must get rapidly smaller as the separation of the atoms increases – the blue curve in the figure shows approximately how the force of attraction varies with distance. It will be zero at large separations. Similarly, the force of repulsion falls off as distance increases. Note that this fall-off is more rapid than for the attractive force, so atoms still attract each other after the repulsion has approached zero.
We must add together the attractive and repulsive forces to find the net force at any separation. This is the same as adding the two curves together. The resulting force-separation curve will depend on the relative sizes of the repulsive and attractive forces. In the figure below, the final force-separation curve, the purple curve, is a good representation of the force between a pair of adjacent atoms.
What can we tell from this curve?
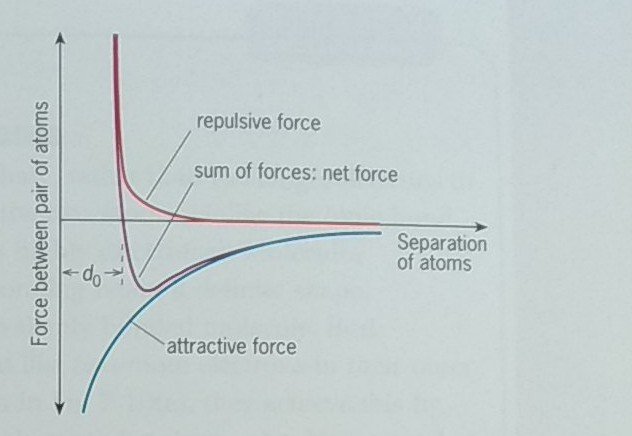
- At a separation d0 the net force between the two atoms is zero. At this separation, the repulsive force exactly balances the attractive force. This will be the equilibrium position of the two atoms. At this separation, they remain stable.
- At very large separations the force between the atoms approaches zero. But the force of attraction does start to have an effect when approaching particles are relatively far apart.
- There is a large force of attraction for separations slightly greater than d0. This attractive force must be overcome if we wish to separate the atoms.
- If the two atoms are brought closer together than d0, the force of repulsion becomes very large.
Pulling atoms apart or pressing them closer together is similar to extending or compressing a spring. This is why we can apply Hooke's law both to extending springs and to deforming solids. If we pull the atoms apart against the attractive force trying to hold them together, we must do work. This is like doing work to raise a mass upwards in a gravitational field. Just as the mass being raised in the gravitational field gains potential energy, so these two atoms gain potential energy as they separate.
We can plot the potential energy gained by these atoms as their separation changes. As is usual, we set the potential energy to be zero for very large separations. When the atoms are moved closer together, the potential energy drops, just as the potential energy of a mass decreases as it falls to Earth. Since it decreases from a zero value, the potential energy must become more negative. The equilibrium position d0 where the net force on the atoms is zero, corresponds to the minimum in the potential energy curve. For a very small change of separation from d0, the potential energy does not change. Take care - the potential energy minimum does not correspond to the minimum on the resultant purple force curve in the figure. The minimum on the force corresponds to the separation where there is the biggest net attractive force, not zero net force.
To decrease the separation further, the atoms must now be moved against the force of repulsion. The potential energy starts to rise again. Eventually it reaches a very large positive value. The type of force-separation curve we have looked at is very helpful for describing the interaction between atoms. Atoms in solids bond together in different ways, and the precise form of the interaction will vary according to the type of bonding involved. How the form of the potential energy curve can be related to the properties of materials and a summary of the main types of bonding found in solids are given below:
The potential energy curve and properties of materials
We have seen that at equilibrium the separation of two atoms corresponds to the position of minimum potential energy. The atoms will remain at separation d0 provided the temperature is at absolute zero. (Later we will discover that reaching absolute zero is not quite possible because of the 'uncertainty principle').
If we give the atoms some energy by heating them to a finite temperature T1, the potential energy (PE) increases to a value PE1.The atoms are bound together by a potential energy ε0 equal to the maximum depth of the curve. The energy that heating gives to the atoms makes them vibrate towards and away from each other, in response to the repulsive and attractive forces. We have pictured the distance between the atoms as a spring between two points on the curve at T1. These two points represent the minimum and maximum separations of the atoms at that temperature.
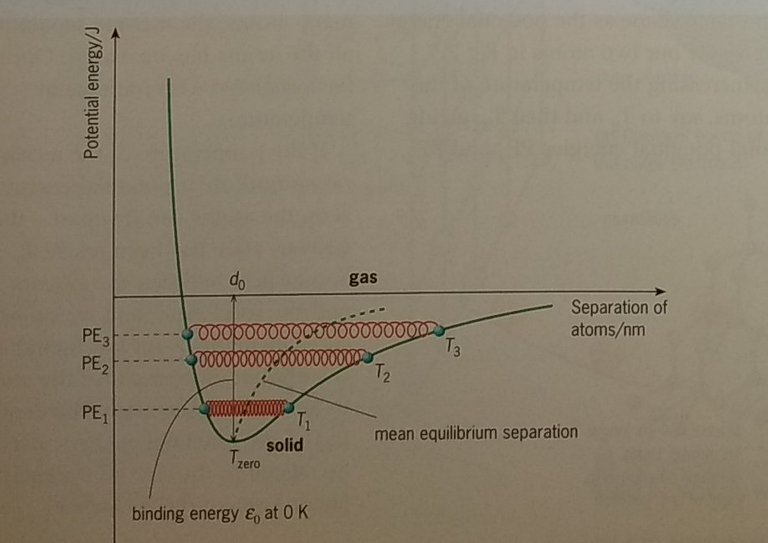
Alternatively, we can think of a marble oscillating backwards and forwards in a bowl within a gravitational field. The more energy we give the marble, the bigger the oscillations as it rises further up either side of the bowl. It is quite helpful to think in terms of an oscillating marble in a bowl that has the same shape as the potential energy curve for our two atoms.
Increasing the temperature of the atoms, say to T2 and then T3 giving total potential energies PE2 and PE3 respectively, makes them vibrate relative to each other by larger amounts. This is shown on the diagram as stretched springs at T2 and T3. The higher the energy of the atom pair, the wider their range of separations.
The mean separation of the atoms is the midpoint of these lines of vibration. We immediately see that, because the potential energy curve is not symmetrical about the minimum, the mean separation increases as the temperature increases. Look at the marble oscillating in the similarly shaped bowl. When this happens for many atoms, the average separation of all the atoms has increased. The solid has expanded with increase in temperature.
If the temperature of the atoms is raised until their potential energy is zero, the atoms can fly apart – the gaseous state has been reached. Somewhere between the gaseous state and the solid state, there is sufficient movement for the atoms to be in the liquid state. The depth (ε0) of the potential energy curve will be related to the sum of the specific latent heats of the substance.
Till next time when I will be discussing the various types of atomic bonding and crystal structure, I remain my humble self, @emperorhassy.
REFERENCES
https://byjus.com/chemistry/melting-and-boiling-point/
https://en.wikipedia.org/wiki/Melting_point
https://en.wikipedia.org/wiki/Boiling_point
https://www.quora.com/What-is-the-effect-of-pressure-on-boiling-point-and-melting-point
https://en.wikipedia.org/wiki/Sublimation_(phase_transition)
https://www.thoughtco.com/definition-of-sublimation-phase-transition-604665
https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/atoms-and-molecules
https://www.khanacademy.org/science/biology/chemistry--of-life/elements-and-atoms/a/matter-elements-atoms-article
https://courses.lumenlearning.com/earthscience/chapter/atoms-to-molecules/
https://study.com/academy/lesson/what-are-atoms-molecules-definition-differences.html
https://www2.estrellamountain.edu/faculty/farabee/biobk/BioBookCHEM1.html
https://www.academia.edu/28756360/Potential_Energy_Curves_and_Material_Properties
https://www.scirp.org/html/3945.html
https://www.researchgate.net/publication/274434771_Potential_Energy_Curves_Material_Properties
Congratulations @emperorhassy! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.