ENERGY CONSUMPTION AND CLIMATE CHANGE: Are we changing the climate?
There is now little doubt that the average global surface temperature is on the increase. What is less certain is how much of the increase is caused by human profligacy, as we consume increasing amounts of energy and generate large amounts of carbon dioxide that passes into the atmosphere. How much does changing temperature change the world’s climate? Were the UK floods of 2007 or the large-scale damage produced to New Orleans in 2005 as a result of a serious hurricane caused in any way by changing temperature and indirectly by human effects on the climate. However, temperature fluctuations have occurred naturally in the past. The UK has had hotter periods when wine-growing was prevalent and colder periods when the Thames froze and was used by skaters. Certainly, as the global temperature rises today, map makers need to redraw the extent of many lakes and coastlines. The potential consequences for the human race are huge.

We have seen how physics has helped us to explore and understand the very small and the very large, from nanoscale electronics to the Universe. But one of the biggest issues facing us today is the changing climate. This is a system of intermediate size but of incredible complexity. Understanding how temperature and climate are influenced is an inter-disciplinary topic in which physics plays an important role. In particular, we need to know just how much we are affecting the climate and how we might reduce the effects despite an ever-increasing population. In this post, I will compare the Earth’s energy sources, the greenhouse effect, and why it is important. I will also compare ways in which we obtain our energy for power and heating.
ENERGY FROM THE SUN
There are three main factors that determine the surface temperature of any planet in the Solar System: The radiation received from the Sun, the energy released internally in the planet (mostly due to radioactivity) and the energy radiated away from the planet into space.
By far the biggest contribution to global energy comes from the Sun. The energy radiated from the Sun peaks in the visible part of the spectrum, and its total quantity is 3.8 × 1026 W. Of this, the Earth receives a tiny fraction, 1.353 kW m-2, a quantity called the solar constant, S. But the Earth is not a perfect absorber: about 30% of the visible radiation is reflected by clouds, ice, the sea and land. This reflected proportion is called the albedo, a, of the Earth: a = 0.30.
The radiation energy reaching the Earth heats the ground, the sea and the atmosphere. It evaporates water and powers the water cycle. However, the atmosphere is too ‘transparent’ to solar energy to be significantly warmed directly by solar radiation; it is warmed by contact with the ground. The heated, moving atmosphere then shares out the absorbed energy more evenly across the Earth: air heated near the ground in the tropics moves in convection currents towards latitudes near the poles. Convection in the oceans also helps to redistribute energy over the Earth. The Gulf Stream, for instance, brings warm water from the Caribbean area to western Europe.
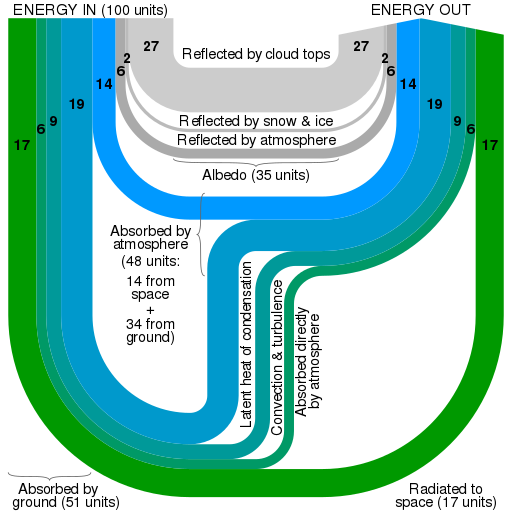
The figure above shows what happens to the incoming energy on Earth. The warm Earth also radiates energy into space. The energy flows are represented by arrows and the figure is an example of a Sankey diagram. The widths of the arrows are proportional to the flow quantities. Such diagrams are used in other examples of flow. An equilibrium is established of a steady mean surface temperature because the power radiated equals the power delivered by the Sun’s radiation.
We can estimate the effect of the atmosphere on the temperature of the Earth’s surface in a calculation in which we assume that the Earth is ‘bare’, acting as a ‘black body’, without an atmosphere. From this, the temperature of the bare Earth’s surface is: T = 254 K or -19 ˚C. But the Earth is not bare, and the actual mean surface temperature is 288 K or +15 ˚C. A small part of the extra 34 degrees is due to an outflow of energy from the interior of the Earth, but most of it is a result of the Earth having an atmosphere.
The emissivity of the Earth’s surface is important and can vary widely. In the most extreme desert regions, for instance Chile at 315 K, the emissivity is 550 W m-2, whereas in a certain part of Indonesia where there are deep convection systems and the temperature can fall to 180 K, the emissivity can be as low as 60 W m-2. The surface heat capacity Cs is defined as the energy required to raise the planet’s surface by one degree and has units of J m2K-1
THE ATMOSPHERIC BLANKET
Molecules in the atmosphere contain energy in various ways. One is that they have translational kinetic energy, as they move through space. Another is that they may spin and so have some rotational kinetic energy and finally, they also have vibrational energy, alternately potential and kinetic as the atoms in molecules oscillate to and fro.
As a rough rule, the temperature of a gas is proportional to the mean translational kinetic energy of its molecules. However, the energy that is important in making the atmosphere a ‘blanket’ for radiation from the Earth is the vibrational energy of molecules. Molecules can increase their vibrational energy by absorbing photons carrying exactly the right amount of energy to increase the vibrational energy by one quantum.
The energy required by a carbon dioxide molecule to jump up a quantum level happens to have a frequency that is close to the peak of the radiation emitted by the warm Earth, at 2 × 1013 Hz (infrared, at λ = 15 μm). Other gases can absorb energy in much the same way: ozone (O3) requires energy at a photon frequency of 3 × 1013 Hz (λ = 10 μm), and water at 4 to 5 × 1013 Hz (λ = 5 μm). A quick reminder here; Ozone is formed from atmospheric oxygen (O2), which is split up by ultraviolet radiation from the Sun and then recombines as O3. There is too little ozone to have a thermal effect as great as carbon dioxide’s but as it also absorbs in the ultraviolet region, it cuts down this dangerously ionizing radiation from the Sun.
THE GREENHOUSE EFFECT
The energy absorbed by a carbon dioxide molecule is re-emitted almost immediately as an infrared photon. Now, all the photons absorbed by carbon dioxide were travelling outwards from the Earth. But roughly half of the re-emitted photons are moving back to Earth. Remember that the incoming radiation from the Sun has an energy peak in the visible part of the spectrum. The atmosphere is mostly transparent to this part because energy absorption by atmospheric gases is at the peak of the radiation emitted by the warm Earth, the atmosphere acts as a blanket. This is like the way that air in greenhouses is kept warmer than air outside: sunlight and short-wave infrared can get through the glass, but the longer wave infrared that is reradiated from the interior is absorbed within. Hence, energy absorption in the atmosphere is called the greenhouse effect.
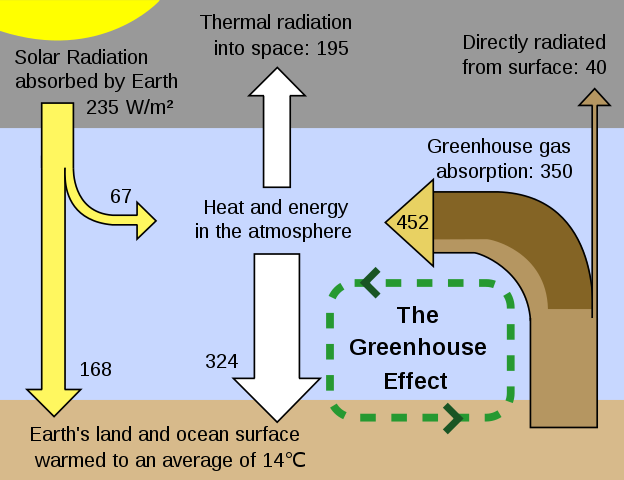
The result is that only about 60% of the thermal radiation from Earth gets through into space, that is, the transmission factor b = 0.6. The energy balance in watts can now be recalculated and equating these gives: T = 289 K or 16 °C.
The predition from this simple model is close to the measured value for the mean surface temperature of Earth, which is 288 K or 15 °C. Not all gases are greenhouse gases, For absorption of infrared radiation by molecules, there need to be bonds between atoms of dífferent elements. So carbon dioxide, water and methane are all examples. Their abundance in the atmosphere is important and so is their effectiveness. Water is 0.5% of the atmosphere and contributes 60% of the greenhouse efect. Carbon dioxide is currently 0.36% of the atmosphere but the molecules have a larger effect. The amount of methane in the atmosphere is small but the molecules are eight times as effective as those of carbon dioxide. In an illustration showing the relative amount of thermal radíatíon passing through the atmosphere within the infrared region as measured by an orbiting satelite, it’s observed that there are dips in the curve which correspond to absorption by greenhouse gases.
MORE ABOUT THE GREENHOUSE EFFECT, AND THE CARBON DIOXIDE BALANCE
Although carbon dioxide is just 0.036% of atmospheric gases, four billion years ago the atmosphere was probably 98% carbon dioxide, rather like the atmosphere of Venus today. The Earth was then cool enough for water to exist as a liquid. Carbon dioxide is water soluble, so rain washed it out of the atmosphere, and chemical processes trapped it as carbonate in rocks such as chalk, limestone and dolomite.
The oceans still contain a large mass of carbon dioxide dissolved in them. Animals in plankton, the sea organisms with the largest total mass, make their shells from carbon dioxide. These eventually fall to the sea floor and become sedimentany rocks. The oceans are therefore a sink for carbon dioxide – they remove the gas from the atmosphere. Over a long time, some of this is recycled as the ocean crust goes deeper into the Earth by the process of subduction. There, it is melted, heated carbonates release carbon dioxide, and so subduction is a source of carbon dioxide. The gas emerges into the atmosphere through volcanoes.
A quicker recycling process than plate tectonics involves organisms,. Plants take carbon dioxide out of the atmosphere, use the carbon and release oxygen. The oxygen is used by most organisms in respiration and re-emerges combined with carbon as carbon dioxide. Dead plants and animals are a sink for the carbon from carbon dioxide if they become fossilised without oxidation. This has happened many times on the Earth, providing large stores of carbon combined in the hydrocarbons of coal, gas and oil.
THE CARBON DIOXIDE BALANCE
About 30% of the carbon dioxide is continuously moving into and out of the atmosphere by various processes, but over long periods of the Earth’s history, oxygen and carbon dioxide have been in balance, in a selfregulating feedback system. Suppose there is an excess of carbon dioxide. This is good for plant growth, so plants take up more carbon dioxide and release more oxygen, restoring the balance. If an excess of oxygen is produced, fires start more easily, so burning vegetation kill plants and put more carbon dioxide into the atmosphere as a combustion product.
CARBON DIOXIDE AND GLOBAL WARMING
We are concerned nowadays about the greenhouse effect because there has been a steady increase in carbon dioxide levels over the last century or so. This is largely due to human activity – the burning of fossil fuels and the increase in the number of farm animals. Both result in more carbon dioxide emission. Farm animals also produce methane, which is an even more effective greenhouse gas.
The consequence of increased carbon dioxide levels is global warming. There has been both global warnming and cooling in past ages, with warm (interglacial) periods or cool periods (ice ages) lasting for thousands to tens of thousands of years. How do we know about these temperatures? Geological and fossil records are a good indication. However, ice cores taken deep into the Antarctic ice give a clear record of atmosphere composition and therefore of the mean global temperature. Ice cores have been drilled at the Russian Antarctic base to depths corresponding to 420 000 years.
To be continued……..
Thanks for reading.
REFERENCES
- https://www.sciencedirect.com/science/article/pii/S1364032114002718
- https://www.econjournals.com/index.php/ijeep/article/view/96
- Climate impacts
- https://phys.org/news/2015-12-sun-energy.html
- https://www.need.org/Files/curriculum/guides/EnergyfromtheSunStudentGuide.pdf
- https://www.esrl.noaa.gov/gmd/education/info_activities/pdfs/LA_life_in_a_bag.pdf
- https://climate.nasa.gov/news/2914/the-atmosphere-earths-security-blanket/
- https://en.wikipedia.org/wiki/Greenhouse_effect
- https://www.britannica.com/science/greenhouse-effect
- Climate change and climate science.
- https://earthobservatory.nasa.gov/features/CarbonCycle
- https://www.britannica.com/science/global-warming/Carbon-dioxide
- https://climate.nasa.gov/vital-signs/carbon-dioxide/
- Global warming: nrdc
hello @emperorhasy very wide and interesting its content I have had the opportunity to read several articles on about global warming and how carbon dioxide in the atmosphere has increased considerably and that even though methane is stronger the highs Concentrations of dioxide make it more lethal. As for methane, I believe that its production in the livestock sector is due to poor management of animals in production units, especially when confined, as their stomachs generate high amounts of that gas. Excellent article, waiting for its continuation.
Hello, @amestyj. Thank you for the great contribution. I really appreciate your coming by and I'm glad that you found the article pleasing.
Yes, poor management of animal production emits methane gas
Also, The dungs they drop when used as manure for crops also have iota of methane content
Hello friend, no doubt cattle manure can generate small amounts of methane gas, in some production units are starting to use digesters for the use of biogas generated by excreta, used for cooking among other functions. Thank you for the interaction friend !
Interesting info
Thank you
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app, which gives you stronger support. Including @stemsocial as a beneficiary could yield even more support next time.
Congratulations @emperorhassy! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!